Discriminatory Disease
Exploring the gender gap in multiple sclerosis
In the last century, women have made great strides, approaching men’s status in the home, political realm, and the workplace. But the body does not follow societal trends, and one form of inequity is getting worse instead of better: Women are twice as likely as men to develop multiple sclerosis (MS), and this disparity has been increasing for at least 50 years (Alonso and Hernán, 2008; Chao et al., 2011; Nashold et al., 2009). MS causes the immune system to rise up against the body and slowly destroy the very tissue it was meant to protect. This mutiny seems particularly tied with womanhood; although the gender ratio of MS is close to 1:1 in children, those numbers skew dramatically in adulthood (Banwell et al., 2007). Relapsing-remitting MS (RRMS), the most prevalent form of the disease, is also the type with the greatest gender imbalance—and it is the incidence of RRMS that has been increasing over time (Ramagopalan et al., 2010). By comparison, primary-progressive MS (PPMS), characterized by slow, steady degeneration without relapses, has no significant gender gap (Tremlett et al., 2005, National MS Society's web page on PPMS), and its rates remain relatively stable (Ramagopalan et al., 2010).
Many scientists invoke sex hormones to account for the gender ratio, yet a state that is quintessentially feminine—pregnancy—reduces relapses far more effectively than any drug on the market. This paradox is driving researchers deep into the details of how estrogen and other sex hormones influence the disease’s course. Hormones, however, are unlikely to fully explain the gender gap, so scientists are seeking factors that promote MS, considering everything from smoking to lack of sun exposure as they tease apart the complex web of factors that underlie the ever-growing female susceptibility to MS.
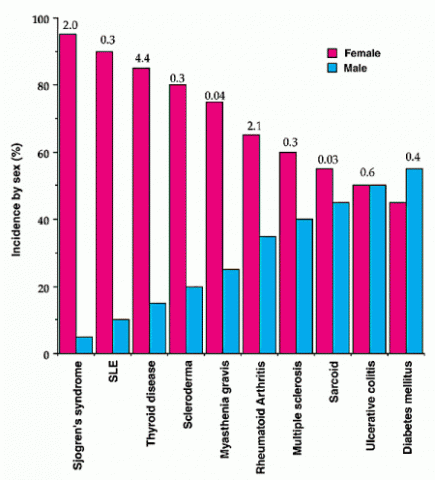
The sex disparity in MS mirrors that seen in many autoimmune disorders. For example, autoimmune thyroiditis, systemic lupus erythematosus, and Sjögren’s syndrome claim a patient population that is more than 80% female (Whitacre, 2001), and overall, nearly 80% of autoimmune diseases disproportionately affect women (Gleicher and Barad, 2007). Scientists have explained this trend by pointing out that female sex hormones stimulate inflammatory pathways, which can exacerbate autoimmune conditions. Women tend to mount a stronger defense against microbial attack than men do; they also produce more CD4+ lymphocytes—a type of white blood cell that activates and directs other immune cells—and larger amounts of proinflammatory cytokines, signaling molecules that call immune responses into action (Whitacre, 2001). In particular, estrogen shifts cytokine signaling to activate the aggressive Th1 and Th17 arms of the immune system (Straub, 2007). Notably, many MS therapies work by reducing Th1 or Th17 responses, thus countering estrogen’s effects (Voskuhl, 2011).
Scientists are starting to get a detailed handle on gender-related differences in the immune response. In female and castrated male mice, for example, T cells that have been activated with a portion of myelin spur an inflammatory response in astrocytes, cells that perform several key functions in the brain; analogous T cells from unaltered male mice, however, do not (Brahmachari and Pahan, 2010). Furthermore, the presence of a particular molecule on the T-cell surface—one of the so-called integrin proteins—correlates with this effect and might underlie it. This finding could potentially reveal a mechanism of sex-specific inflammation that operates in MS.
If estrogen has a proinflammatory effect, then pregnancy—when sex hormones are at their peak—might be expected to dramatically worsen autoimmune diseases. However, the opposite is true: Pregnancy improves most autoimmune conditions, including MS, rheumatoid arthritis, and psoriasis. It cuts acute MS attacks by 80%, a potency comparable to that of the most effective drug on the market, whereas first-line treatments claim only 30% to 50% relapse reduction (Polman et al., 2006; Voskuhl, 2011). Sex hormones therefore seem to play opposing roles in MS: At normal concentrations, they promote inflammation; at high concentrations, they dampen it (Papenfuss et al., 2011).
Estrogen’s puzzling role reversal makes sense from an evolutionary perspective. Immunosuppression is integral to creating a healthy baby; the fetus carries paternally derived molecules, and the mother’s immune system must not attack the unfamiliar body (Wegmann et al., 1993; Hill et al., 1995). High estrogen concentrations curb production of proinflammatory cytokines and stimulate the Th2 arm of the immune system, which keeps inflammation in check (Papenfuss et al., 2011; Matejuk et al., 2001). Recent research suggests that estriol, a hormone secreted by the placenta and present in significant quantities only during pregnancy, promotes the growth of tolerogenic dendritic cells, immune cells that can limit inflammation (Papenfuss et al., 2011). Furthermore, some pregnancy-associated sex hormones might help the fetus in other ways: Estrogens, progesterone, and prolactin are believed to be neuroprotective. Progesterone might help damaged neurons remyelinate, and estrogen-related compounds have been shown to protect against various animal models of neurodegenerative disease. In addition to aiding the developing brain in utero, these chemicals might help repair some of the damage wrought upon the nerve cells of MS patients (Voskuhl, 2011; Spence et al., 2011).
Researchers are beginning to unveil the subtleties of these activities. Investigators have found, for example, that a particular estrogen receptor, ERβ, influences the behavior of microglia, immune cells of the brain and spinal cord that play a prominent role in driving MS. Some estrogen-related compounds curb microglial activity through the receptor, although others interfere with this anti-inflammatory effect (Saijo et al., 2011). The results suggest that different relative concentrations of such hormones might ramp immune activity up or down. Other work, too, is uncovering how estrogen receptors in particular cell types might suppress inflammation; for example, one report showed that a second receptor, ERα, seems to exert its beneficial effects through astrocytes, but not neurons (Spence et al., 2011). The explanation for estrogen’s disparate effects—inflammation, anti-inflammation, and neuroprotection—likely lies in the many different cells and receptors it influences.
Unfortunately, pregnancy only temporarily relieves MS symptoms; in the first few months after giving birth, the relapse rate increases dramatically as hormone levels return to normal (Lee and O’Brien, 2008). However, recent research suggests that breast-feeding might extend the beneficial effects. Langer-Gould and colleagues tracked 32 women for a year after they had given birth and found that 87% of new mothers who didn’t breastfeed experienced relapses within 2 months. By comparison, only 36% of women who breast-fed experienced relapses within the same time frame (Langer-Gould et al., 2009). Another study documented similar effects, although its results did not reach statistical significance (Hellwig et al., 2009). The authors suggest that benefits from breast-feeding arise from hormones; prolactin, which promotes lactation, is thought to enhance myelination and myelin repair, thus counteracting the damage MS causes to myelin, the fatty sheath around neurons that allows them to work properly (Langer-Gould et al., 2009). However, prolactin is only part of the story; through the action of several hormones, breast-feeding induces amenorrhea, which the authors speculate might protect against MS: Onset of MS is rarer in men, postmenopausal women, and prepubescent girls than it is in menstruating women. Therefore, breast-feeding, like pregnancy, might alter the course of MS in a sex-hormone-dependent way.
However, the implications of these findings are controversial. They suggest that female MS patients should breastfeed after birth, but doctors generally advise MS patients to abstain from breast-feeding so they can resume their drug treatments. Patients on MS medication should not breastfeed, as the chemicals might be transmitted through breast milk and if so, might harm the infant (Langer-Gould et al., 2009).
Furthermore, not everyone agrees that breast-feeding is beneficial: Some research groups have failed to find a correlation between breast-feeding and relapse rate (Vukusic et al., 2004; Neuteboom et al., 2011; Portaccio et al., 2011). The largest study on the subject—which analyzed 302 full-term pregnancies—concluded that breast-feeding did not protect against relapses (Portaccio et al., 2011). Small sample sizes have hindered most studies that have attempted to probe this issue (Kieseier and Wiendl, 2010), and detractors of the breast-feeding hypothesis suggest that self-selection bias has warped some of the results: They argue that mothers with more severe MS are more likely to return immediately to treatment than those with milder disease, and breast-feeding seems protective simply because women who choose that route were better off to begin with (Airas et al., 2010; Portaccio et al., 2011). Although this argument holds up for several studies, the findings of Langer-Gould and colleagues can’t be explained away by self-selection bias, as breast-feeding and non-breast-feeding mothers had similar measures of disease severity (Lander-Gould et al., 2009; Kieseier and Wiendl, 2010).
The experimental inconsistencies could also result from differences in study design, as they differed in duration and rules for group assignment. For instance, the Langer-Gould report included only women who breast-fed exclusively, whereas others included in the “breast-feeding” group women who alternated between breast-feeding and formula feeding; this practice might have obscured benefits of nursing (Langer-Gould et al., 2009). Similarly, the Portaccio work combined women who had breast-fed for less than 2 months with those who did not breastfeed at all. Other studies lasted for different lengths of time and were conducted in different countries, which had varying cultural expectations about breast-feeding that could introduce bias (Kieseier and Wiendl, 2010). Ultimately, research on the subject is hindered by an unavoidable feature: These are observational analyses. Patients choose whether to breastfeed; they are not randomly assigned to one group or the other. Larger, more standardized studies should help resolve the debate, but in the meantime, scientists agree that breast-feeding doesn’t aggravate MS; among women who refrain from treatment just after birth, relapse rate is no worse in breast-feeding mothers than it is in non-breast-feeding mothers (Vukusic et al., 2004).
Instead of investigating how pregnancy—and perhaps breast-feeding—might protect against MS, some researchers have focused on men to understand potential sex-related factors that give them an advantage over women against the disease. Not only are men less likely to develop MS, but they also tend to get it later in life, at 30 to 40 years old; in contrast, women become sick at around age 18 to 40 (Voskuhl, 2011). This observation, which also applies to other autoimmune diseases such as rheumatoid arthritis, might stem from testosterone’s anti-inflammatory properties. Notably, 24% of male MS patients have significantly less testosterone than healthy controls of the same age (Wei and Lightman, 1997)—and quantities of the hormone decline with age, which might help explain the later age of MS onset in men (Voskuhl, 2011). Experiments that probe potential mechanisms of testosterone’s action indicate that it soothes the immune system by promoting a Th2 response in vitro and in mice with experimental autoimmune encephalomyelitis (EAE), a condition that shares some features with MS. Testosterone suppresses production of inflammatory cytokines and, in doing so, prevents men from mounting a strong immune response (Dalal et al., 1997; Gold and Voskuhl, 2009).
Men are therefore privileged when it comes to autoimmune conditions because they have testosterone and lack estrogen, a hormonal state that gives them weaker immune systems than women. This advantage is not absolute, however: Some autoimmune diseases, such as type 1 diabetes, psoriasis, Wegener’s granulomatosis, and ankylosing spondylitis, affect men at least as commonly as women.
Recognizing that the possible connection between MS and sex hormones could be harnessed for medical use, researchers have attempted to treat the disease with hormone-replacement therapy. Some of the findings have been disappointing: Birth-control pills, which mimic the hormonal profile of pregnancy, don’t seem to protect against MS. Researchers have proposed that the drugs contain the wrong balance—and an insufficient dose—of sex hormones (Voskuhl, 2011). Other investigations, however, have yielded more optimistic results. In a current study, women with MS are adding estriol—a pregnancy-specific hormone—to the MS medicines that they are taking. Preliminary results look promising (Voskhul, 2011). Another trial, POPART’MUS, aims to extend the beneficial effects of pregnancy by treating women with high doses of progestin and physiological doses of estradiol immediately after delivery, and then continuing the agents for 3 months (Vukusic et al., 2009). Testosterone also seems to hold some hope. In a small study, testosterone treatment improved symptoms of male MS patients who were observed without treatment for 6 months and then given testosterone for 1 year. Cognitive performance—assessed with a test that measures working memory, addition skills, and attention span—improved significantly during the treatment period. Furthermore, the rate of brain atrophy slowed by 67%. However, there was no measurable effect on lesions (Sicotte et al., 2007).
Beyond hormones
Although hormones might explain much of the gender gap in MS, other influences likely contribute as well. Environmental and genetic factors shape risk for the illness in general and might also shift the sex balance. Some scientists are pointing to environmental cues in particular, as RRMS—the type of MS with the largest gender gap—displays the most seasonal fluctuation. People with RRMS are far more likely to have been born in the spring than in the fall—for example, they have 1.43 times more May than November birthdays; in contrast, PPMS patients are only 1.15 times more likely to have a May birthday (Sadovnick et al., 2007). Thus, this disease seems to arise partly from external stimuli that vary over the course of a year, biasing a developing fetus toward developing MS (Nashold et al., 2009; Ramagopalan et al., 2010), and researchers suspect that other environmental factors might act during later stages of life. For all of these reasons, investigators are searching for influences that affect the gender distribution from outside the body as well as within it.
One such influence could stem from behavioral differences between boys and girls. The “hygiene hypothesis” suggests that fastidious children run a greater risk of developing autoimmune diseases later in adulthood than their playmates. Exposure to microbes in the first few years of life renders individuals less vulnerable to MS, the thinking goes. Girls tend to be held to higher standards of cleanliness than boys are and might suffer long-term consequences from this social conditioning (Clough, 2011). So although boys may horrify their parents by eating bugs or refusing to wash their hands, they could be protecting themselves against autoimmune diseases down the line. The hygiene hypothesis ties in neatly with another branch of MS research: Some scientists believe that viral infections after childhood act as a trigger for MS (see "Viral Villain"). In this scenario, early exposure provides protection from microbes that can trigger autoimmunity if they’re encountered for the first time during adolescence or early adulthood (Kakalacheva et al., 2011; Wingerchuk, 2011).
Even if hormones and hygiene can explain much of the gender gap in MS, a troubling issue remains. Although women have made many gains in the last 50 years, the sex ratio for the disease has been growing steadily during that time period. At the turn of the 20th century, men and women contracted MS at nearly equal rates in North America; today, about twice as many women as men have the disease, and female prevalence continues to rise. In some countries the ratio started at 1:1 in the 1950s and now approaches 4:1 (Alonso and Hernán, 2008; Chao et al., 2011). There is no evidence that the gender gap is widening in other autoimmune illnesses that disproportionately affect women (Cynthia Crowson and Sherine Gabriel, personal communication), so whatever accounts for the growing discrepancy must relate to something other than general mechanisms of self-reactivity.
Some researchers have argued that gender-associated changes in behavior are driving the increasing disparity in MS. Smoking became more common among women during the 20th century, starting with rebellious flappers who challenged gender norms by flaunting cigarettes (Brandt, 1996); conversely, smoking among men declined over the same time period (Palacios et al., 2011). Smoking increases the risk of developing MS (Wingerchuk, 2011) and speeds the rate of disease progression after a person experiences his or her first neurological symptom (Wingerchuk, 2011). The habit is thought to disrupt immune regulation, as smoking increases the risk of many autoimmune disorders in addition to MS (Costenbader and Karlson, 2006)—although if lighting up contributed significantly to the widening gender gap through this mechanism, one would expect that other autoimmune disorders would reflect a similar trend. A study in 2011 found a strong correlation between the changing sex ratios in smoking prevalence and MS (Palacios et al., 2011). However, it cannot fully explain the phenomenon, according to the authors’ calculations, nor can it account for the gender inequality in general, as fewer women than men smoke (American Lung Association). Furthermore, shifts in smoking behavior would be expected to tilt the sex ratio by causing a decline in MS incidence among men (Simon et al., 2011)—and yet male MS rates remain stable while female MS rates rise (Ramagopalan et al., 2010). Although smoking might contribute to the changing sex ratio, another environmental factor must also be at play.
Sun exposure is also a candidate, as it causes the body to produce vitamin D, which is thought to protect against MS (Nashold et al., 2009, and "The Sunshine Suspect"). Vitamin D has immunomodulatory properties (Wingerchuk, 2011) and can ameliorate features of EAE (Nashold et al., 2009)—and recent research suggests that estrogen helps mediate the substance’s beneficial effects (Correale et al., 2010; Nashold et al., 2009). Oral vitamin D3 supplements protected normal female mice and ovariectomized females that received estrogen supplements from onset of EAE. However, mice without estrogen—males and ovariectomized females—succumbed to the disease (Nashold et al., 2009). Perhaps lifestyle changes, such as spending more time indoors or increased use of sunscreen, could disproportionately affect women, thus altering the gender ratio of the disease (Handunnetthi et al., 2010).
MS stems not from any single source or type of influence but rather from interactions among genes and environmental contributors—and some of these synergies might increasingly target women. One gene family, implicated as a major player in almost all autoimmune diseases, including MS, could well participate in such a scenario (Chao et al., 2011). The major histocompatibility complex (MHC) of mice presents molecules to immune cells, a process that, among other things, helps distinguish self from nonself entities. Particular versions of MHC-family genes (called HLA genes in humans) increase the risk of autoimmune disease, and certain forms of an MHC gene named HLA-DRB1 are strongly associated with MS. Some work suggests that the HLA-DRB1*1501 variant seems to confer a higher risk of MS for women than for men (Irizar et al., 2011; Chao et al., 2011). Furthermore, the prevalence of HLA-DRB1*1501 is increasing in women with MS but remains stable in male MS patients; in families with MS, affected nieces are more likely to have the HLA-DRB1*1501 allele than their affected aunts, according to one study (Chao et al., 2009). These observations suggest that the risk that HLA-DRB1*1501 confers on women seems to be increasing—perhaps, the authors suggest, due in part to some environmentally induced modification to the gene (Chao et al., 2009; Chao et al., 2011).
Environmental stimuli that act on HLA-DRB1*1501 might also be changing and thus disproportionately affecting women (Chao et al., 2011). Vitamin D increases HLA-DRB1*1501 gene activity, for instance (Ramagopalan et al., 2009), and researchers are proposing explanations for how reduced vitamin D levels—perhaps from spending more time indoors or using sunscreen—might be tilting the gender ratio through the MHC (Handunnetthi et al., 2010, and "The Sunshine Suspect").
Inquiry about why women are more susceptible to MS has turned into something of a Pandora’s box, providing more questions than answers. Discrepancies in the breast-feeding studies, the observation that birth-control pills don’t confer the protective effects of pregnancy, and attempts to identify the myriad environmental factors that might be driving the changing sex ratio illustrate just some of the unresolved issues. Insight into these matters will likely provide clues about not only the gender gap in MS, but also general mechanisms of the disease and possible approaches to cures or preventive strategies. These advances might do more than reduce the discrimination of the disease, as they could one day benefit men and women alike.
Key open questions
- Given that autoimmune diseases share some striking features—they disproportionately affect women and abate during pregnancy—could a treatment that works for other autoimmune diseases be co-opted to treat MS?
- The sex ratio in the United States remains around 2:1, but in Canada and Ireland it approaches 4:1 (Chao et al., 2011). What cultural, behavioral, environmental, and/or genetic factors could account for the different sex ratios in different countries?
- If breast-feeding does indeed protect against MS, why do its beneficial effects seem to last for only 3 months (Hellwig et al., 2009), considering that the hormonal state of breast-feeding lasts as long as a baby is nursing?
- Studies on the effects of breast-feeding in MS patients vary widely in their conclusions, possibly due to differences in patient populations or study design. How might these differences contribute to the conflicting results? What were the breast-feeding habits of the women who benefited from breast-feeding?
- Vitamin D and estrogen might work synergistically to counteract MS (Nashold et al., 2009). What other synergistic effects might exist?
- Birth control pills don’t appear to have an impact on MS, possibly because they have the incorrect balance and an insufficient dose of hormones. What would be the proper dose, and would it be safe to take such a medication every day as a prophylactic measure against MS?
- Relapsing-remitting MS is the form of MS with the greatest gender imbalance. How do its pathological mechanisms differ from those in other forms of MS, and why is it so skewed toward women?
- Why is the HLA-DRB1*1501 genotype and penetrance increasing in women?
- How does prolactin repair myelin damage, and does it do so in MS patients? Are its effects immediate?
Note
This article was written for a Stanford class called “Brain and the Immune System.” Students learned about molecular and cellular interactions between the nervous and immune systems, and wrote final papers on a topic in neuroimmunology.
Image credits
Thumbnail on landing page. “Serrai di Sottoguda ‘Emozioni a confronto,’" Roberto Ferrari, 2006. Released under Creative Commons Attribution-ShareAlike 2.0 License CC BY-SA 2.0.
Fig. 1. Reprinted by permission from Macmillan Publishers Ltd: Nature Immunology, Sex differences in autoimmune disease. Whitaker CC, 2:777-780, copyright 2001.
Fig. 2. "Estriol 3D Model," MindZiper/ Wikipedia, 2001. Released under the Creative Commons CC0 1.0 Public Domain Dedication.


