The Sunshine Suspect
Risk II: Homing in on vitamin D
A dearth of sunshine might hasten more than a gloomy mood. In the mid-1900s, researchers noticed that populations living farther north or south had higher rates of MS than those closer to the equator. For example, MS is seven times more prevalent in Tasmania, an island at the southern tip of continental Australia, than in Queensland, the country’s “Sunshine State,” which lies more than 2000 km to the north (McLeod et al., 1994). Geography provided a clue about possible risk factors for the disease, and investigators sought explanations related to latitude—such as climate, diet, sanitation, socioeconomic status, pathogens, and parasites. But interest in most of these candidates faded as studies failed to find evidence that they cause MS. The distribution of the disease cannot be explained solely by genetic variation between populations, either. Tasmanians and Queenslanders have a similar mix of ethnic backgrounds, and the risk patterns of migrants—at least up until a certain age—tend to reflect those of their new homes. For example, people who moved to Sweden from Iran at an average age of 17 developed MS at the higher Scandinavian rate (Ahlgren et al., 2010).
Based on the connection between disease and location, researchers proposed a relationship between sunlight and MS in 1960 (Acheson et al., 1960). After the 1974 discovery that the sun’s ultraviolet rays trigger vitamin D synthesis, scientists began to make the case that the vitamin ties together sunshine and protection from the disease (Goldberg, 1974). An exception to the geographic trend drove the hypothesis: Norwegians who ate a lot of fish—a dietary source of vitamin D—had lower rates of MS than Norwegians who did not. With sun exposure being approximately equal, the argument went, the vitamin D contained in fish might help prevent MS.
People need to consume a tremendous amount of fish to counteract the effects of a dreary winter, however, because dietary sources of vitamin D pale in comparison to those provided by the sun. For example, a 3-ounce fillet of salmon contains about 447 international units (IU) of the compound, a cup of fortified milk has approximately 100 IU, and multivitamins include 400 to 600 IU. In contrast, 20 minutes of beach time (sans sunblock) translates to roughly 10,000 IU (Simon et al., 2011).
The sunshine vitamin has continued to provide tantalizing evidence of protection from MS, while alternative theories to account for the latitude effect have been placed on hold. “I think researchers tend to follow where the data lead them, and right now there is a lot of consistent evidence suggesting that vitamin D is associated with MS,” says Kassandra Munger, an epidemiologist at Harvard School of Public Health in Boston, Massachusetts.
The body uses vitamin D during processes that include bone maintenance, cell proliferation, and immune regulation, but it must transform the vitamin first. When ultraviolet B rays from sunlight hit the skin, they trigger a reaction that produces vitamin D3 (cholecalciferol). It and vitamin D2, the forms also present in food and supplements, are then converted into 25-hydroxyvitamin D (25(OH)D) in the liver. Finally, the kidneys and other tissues turn that substance into a hormone called 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3)—the physiologically active material, which is also called calcitriol. When doctors evaluate patients’ vitamin D concentrations, they use 25(OH)D because it’s the most stable form.
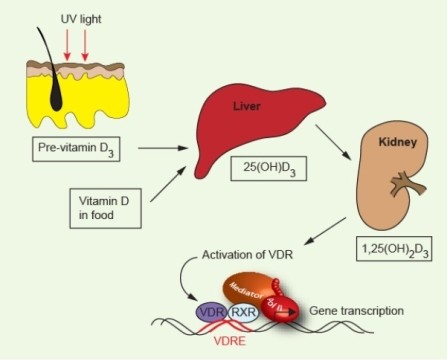
Once investigators gained confidence that vitamin D might protect against MS, they started looking for correlations between 25(OH)D concentrations and the disease. Their results have supported the association: Individuals with the disease, on average, have smaller quantities than do healthy controls. But studying people who already have MS leaves open the possibility that something about the illness underlies the dearth of vitamin D rather than the other way around. If MS patients stay indoors more than healthy individuals, for instance, they’d likely harbor less of the vitamin. To show that a lack of the substance helps trigger the illness, researchers must establish that the vitamin was scarce before disease onset.
In 2004, Munger’s team undertook the daunting task of analyzing dietary questionnaires collected from almost 200,000 women in the United States in 1980 and 1991 as part of the long-running Nurses’ Health Study. A subset of the interviewees went on to acquire MS. Women who had reported taking at least 400 IU of vitamin D per day were 40% less likely to develop MS than were those who did not take supplements (Munger et al., 2004).
In a second study, Munger and her colleagues assessed circulating levels of 25(OH)D in blood samples collected from 771 healthy military personnel before some of them acquired MS (Munger et al., 2006). The researchers divided the recruits into five groups, using 25(OH)D levels among the people who did not develop MS as the basis for the groupings. Then they looked at people who had come down with the disease. White individuals in the highest quintile of vitamin D levels turned out to have a 60% reduced risk of MS compared to white individuals in the lowest quintile. No black and few Hispanic subjects had high levels of vitamin D; therefore, the researchers were unable to determine whether larger concentrations correlated with lower MS rates in these populations.
D’s modus operandi
How vitamin D might keep MS at bay, whether it can help treat people who already have the disease, and how much would be needed for preventive or therapeutic use remains unknown. People deficient in vitamin D (below 20 nanograms of 25(OH)D per milliliter of blood) are at risk of osteoporosis, rickets, and thin, brittle bones—and the nutrient’s role in maintaining skeletal health is fairly well understood. In contrast, although vitamin D participates in a variety of processes, it’s unclear whether more than 20 ng/ml is required to alter these functions in a way that defends against nonskeletal disease.
Because MS involves aberrations in the immune system, researchers have been probing how the vitamin influences cells and pathways there. In general, the active form of vitamin D exerts its physiological effects through the vitamin D receptor, which turns genes on and off when stimulated. A number of vitamin-gene interactions are currently under investigation. For example, vitamin D appears to activate at least one gene variant associated with the disease, HLA-DRB1*1501 (Ramagopalan et al., 2009). In general, evidence suggests that the vitamin tames harmful inflammation; it could do so by rousing the suppressive side of the immune system, by reining in the inflammatory side, or by deploying both mechanisms simultaneously.
In one report, higher quantities of circulating vitamin D correlated with stronger regulatory T cell function in adult MS patients (Smolders et al., 2009). In another study—on cultured T cells from MS patients—the active form of the vitamin, calcitriol, boosted the number of cells that make inflammation-suppressing proteins and slashed the number of cells that make proinflammatory ones (Correale et al., 2009).
Calcitriol also guards against a disease called experimental autoimmune encephalomyelitis (EAE) that mimics some aspects of MS in rodents (Hayes, 2000). Last year, a study on mice with this illness demonstrated one way in which calcitriol dampens inflammation: It binds to a vitamin D receptor that controls the IL-17 gene and thereby curbs production of interleukin-17, a protein that incites immune activity (Joshi et al., 2011). Research also suggests that the anti-inflammatory protein interleukin-10 might be involved, as the vitamin fails to halt EAE in mice that lack the IL-10 gene (Spach et al., 2006). Additionally, calcitriol stunts the production of a protein that directs inflammatory T cells toward the central nervous system—an essential step in the initiation of EAE (Chang et al., 2010).
In patients, these anti-inflammatory effects might avert relapses (Munger et al., 2011). One study found that the amount of circulating vitamin D in children with MS correlates inversely with the number of relapses they suffer (Mowry et al., 2010). Scientists are currently running at least three double-blinded, randomized, controlled clinical trials that involve vitamin D supplements. They aim to determine whether supplements, in addition to MS therapy, can decrease relapses or the number of new and expanding brain lesions in relapsing-remitting MS patients. Thus far, results from a study of 23 patients suggest that a high daily dose of vitamin D provides no therapeutic benefit over a lower dose (Stein et al., 2011). However, the trial does not put an end to the story, as it was small and ran for only 6 months (Solomon, 2011). Merck is sponsoring perhaps the largest trial, called SOLAR, to evaluate the safety and effectiveness of a 6670-IU vitamin D3 dose as an add-on treatment to interferon β-1a (Rebif) in 384 European patients. Researchers should know the results by March 2014 (www.clinicaltrials.gov; NCT01285401). If this work shows that vitamin D stifles relapses, it will mean much to MS patients, but it won’t address whether the vitamin prevents the disease.
Insight into how vitamin D might ward off MS could potentially come from researchers in fields such as type 1 diabetes, asthma, and tuberculosis. Like MS, these diseases involve the immune system and have been linked with vitamin D insufficiency through observational studies and laboratory experiments. Scientists suggest that because vitamin D tempers inflammatory reactions, it could protect against onset or exacerbation of these diseases.
As part of their investigations into vitamin D’s relation to MS, researchers are trying to figure out when the substance’s influence tips the inflammatory balance away from MS. “Vitamin D’s effects on the promotion of suppressor molecules is reasonably well validated,” says Graeme Stewart, an immunologist at the University of Sydney in Australia. “But it is difficult to be certain that this makes a difference in MS because we don’t know when in life the predominant activities of vitamin D occur. Some say in utero, some say in early adolescence, and there’s evidence that it’s an immunomodulator once the disease is ongoing.”
Observational studies have yielded ambiguous results on the matter of timing. Some research suggests that the protective power of vitamin D occurs before adulthood, as older migrants retain the risk of their homeland. On the other hand, some reports suggest that not just young people but also adults can alter their risk by moving to a sunnier locale (Hammond et al., 2000). Studies based on birthdays suggest that the vitamin’s impact begins in the womb. In Scotland, individuals born in April, after the winter, have twice the rate of MS as those born after the summer (Willer et al., 2005). To confirm the gestational association, epidemiologist Alberto Ascherio of the Harvard School of Public Health is sifting through roughly 300 records maintained in Finland on the 25(OH)D levels of pregnant women. More than 40 years have passed since this information was gathered in the 1960s, allowing Ascherio to assess whether the individuals who acquired MS as adults were born to mothers with unusually low concentrations of vitamin D. He says he hopes to have results by 2014.
Taking action
Despite uncertainty regarding its mechanism, vitamin D is currently the best candidate for preventing MS. Unlike investigators’ other leads, its levels can be altered inexpensively with supplements. However, no large, controlled clinical trials have directly tested the hypothesis that supplements can prevent MS or any other nonskeletal disease. These trials are essential, because observational studies don’t allow researchers to draw conclusions about causation; too many confounding factors obscure results. For example, vitamin D might reflect good overall health rather than contribute to it. People who walk to work rather than drive or who participate in outdoor sports might well have higher vitamin D levels than their neighbors who do not, says JoAnn Manson, an epidemiologist at Harvard Medical School in Boston. Health benefits could result from the physical activity rather than the vitamin D, she says.
Without clinical trials or a wealth of well-designed observational studies that can rule out such alternative explanations, a national panel in the United States, put together by the Institute of Medicine (IOM), decided that the evidence is inconclusive about whether a relatively large intake of vitamin D can prevent nonskeletal diseases. Based on the scientific literature on bone health, the panel recommended 400 to 600 IU of vitamin D per day in individuals aged 1 through 70 (400-800 IU in those over 70) in its November 2010 report. In addition, it warned that daily supplements greater than 4000 IU could cause long-term harm. Certain studies suggest that people with high blood levels of vitamin D are at risk of falls and urinary tract stones. Moreover, the panel set an upper limit because the long-term effects of high doses have not been systematically assessed. Even a slight chance of risk would be unacceptable when it comes to advising hundreds of thousands of healthy people.
The report stirred up a storm of criticism. Many researchers countered that higher doses than those recommended by the panel are needed to prevent nonskeletal diseases. They dispute the evidence of harm and argue that observational studies supporting vitamin D’s protective role in MS, certain cancers, asthma, diabetes, tuberculosis, and a dozen other maladies warrant recommendations of at least 1000 IU daily.
The evidence for taking supplemental doses greater than 4000 IU is strong enough, given how simple and safe vitamins are to take, says George Ebers, a neurologist at the Wellcome Trust Centre for Human Genetics in the United Kingdom. He disagrees with warnings that too much vitamin D can be dangerous and advocates increased food fortification. “It’s an equation including costs and benefits,” Ebers says. “For some of us, that equation is easy.” Edward Giovannucci, a nutritional epidemiologist at Harvard School of Public Health, says the panel’s admonition that high-dose supplements could be dangerous was unwarranted, given that it was primarily based on one clinical trial that found harm in a single annual dose of 500,000 IU, which far exceeds what people normally process in one day. The idea behind supplementation, Giovannucci says, is to compensate for what humans no longer make now that outside activities such as hunting and gathering have been replaced with earning money in factories and offices, and spending it in malls and grocery stores.
The IOM report reinforced a realization that had been growing among vitamin D proponents for years: Changing national guidelines on vitamin D intake—and proving that higher doses are safe—would require a randomized, controlled trial. In December 2011, MS researchers from across the globe gathered in Chicago for the first meeting of the “Vitamin D Summit Task Force,” organized by the U.S. National Multiple Sclerosis Society. Their task—to design a feasible trial on vitamin D prevention of MS—presents a daunting challenge. MS might take 30 years to develop after inadequate vitamin D in utero or early childhood, but a 30-year clinical trial is impractical, given the expense. Second, about 7.5 people per 100,000 are diagnosed with the disease each year in the United States, according to a Mayo Clinic report in 2000. Therefore, to show a significant change in the rate of MS, the ideal trial would enroll hundreds of thousands of participants. Again, the larger the trial, the more it costs.
To limit enrollment to several thousand individuals, the group considered the possibility of recruiting people at high risk of the disease: those with siblings or parents who have MS. Finally, because no one knows when vitamin D protects against MS, the researchers discussed dividing participants into age groups and supplementing each group for 5 years. People in the treatment arm would be given 4000 IU daily. Committees that evaluate the ethics of clinical trials should readily approve that dose because it’s no higher than the upper limit set by the IOM, says Gavin Giovannoni, a neurologist at Barts and The London School of Medicine and Dentistry in the United Kingdom and a task force member. However, if approval weren’t a concern, he says, the task force would have tested a higher dose, perhaps 10,000 IU, a dose more likely to produce results. In any case, these ideas will evolve. “It will take 3 or 4 years for us to get the study design sorted out and the funding in place, but we are all very determined,” says Giovannoni (who is also an MSDF scientific adviser). “We have to do this. We think it’s essential to have class one evidence—a randomized, controlled clinical trial—to convince the world to take vitamin D.”
Key open questions
- At what stage or stages of life do high levels of vitamin D help protect a person from multiple sclerosis?
- Can vitamin D supplements prevent MS? If so, when are they most effective?
- Research suggests that vitamin D dampens excessive inflammation. Do people with low levels of vitamin D react differently to infections than people with high levels of vitamin D?
- Does vitamin D strengthen a regulatory T cell response in healthy people? Is this response defective in people who later acquire MS?
- At what dose, if any, do vitamin D supplements become dangerous?
- Can vitamin D prevent relapses in people who have MS? If so, what doses are effective?
Previous article in series: "Whodunit?"
Next article in series: "Viral Villain"
Image credits
Thumbnail image on landing page. “Vitamin D," Elizabeth Lloyd, 2011. Released under Creative Commons Attribution 2.0 Generic License CC BY 2.0.
Fig.1. Courtesy of Sreeram Ramagopalan, Wellcome Trust Centre for Human Genetics.



suggested by George Ebers
This paper is often cited as the origin of the sunshine hypothesis and this is largely true, but it was temporally immediately after Dean and was undoubtedly derived from Dean’s observations in South Africa (Dean, 1949). However, Acheson was an adviser to Dean in this study as acknowledged at the end of the paper. Acheson, who subsequently became the chief medical officer for the U.K. and later dean of the Southampton Medical School, proposed at a Queen Square seminar that MS was related to sunshine deficiency. He was told by FMR Walshe, then the dean of the National Hospital, that it was “more likely to be moonshine,” the implication being that the young Acheson was under its influence.