B Cells Step Into the Limelight
Why MS researchers recast a well-known character
In the body’s theater, the immune system’s show veers off-script to produce multiple sclerosis. Miscues and mixed signals direct cast members to creep into scenes where they don’t belong and to muck up intricately choreographed dances. The ensemble, which normally protects the body, wreaks havoc in the central nervous system (CNS) by stirring inflammatory trouble that trips up brain cells. These slip-ups and stumbles send the plot in new and dangerous directions.
Researchers are trying to figure out how different immune actors bumble the lines and steps. They want to know who is directing the performance, and how the disease’s devastating drama unfolds.
One candidate crew of immune-cells-gone-rogue is B cells, the stars of pathogen tagging. They’re famous for pumping out tiny molecular flags—antibodies—that stick to trespassers, marking them for destruction. In healthy people, B cells bear antibodies that brand mostly bad guys. In MS, however, misbehaving B cells might churn out flags that also latch onto patients’ own tissues.
But B cells could make mischief in other ways as well. They perform many parts besides “growing up to be antibody-producing cells,” says neurologist Robert Lisak of Wayne State University in Detroit, Michigan. Many of these functions could contribute to MS. In the past few years, the seedling idea that antibody-independent roles of B cells could promote MS has not only sprouted, it’s blossomed. In 2008, Stephen Hauser of the University of California, San Francisco, and colleagues showed that rituximab, a drug that wipes most B cells from the bloodstream, drastically cut down on brain lesions and clinical relapses in MS patients (Hauser et al., 2008). Because the drug skips over B cells that make antibodies—and because clinical results often precede drops in preexisting autoantibody levels—Hauser’s study pointed to an antibody-independent role for B cells in MS.
The rituximab finding also helped shift the field’s focus from T cells—another member of the immune cast—to B cells, says Francesca Aloisi, a neuroimmunologist at the Istituto Superiore di Sanità in Rome, Italy. Since its publication 4 years ago, about 600 studies have cited Hauser’s paper, according to Google Scholar. “It switched on something in people’s heads,” Aloisi says, and spurred researchers to think more about B cells. Now, they’re figuring out how rituximab works, tracking misplaced collections of B cells in the CNS, and coming up with novel ideas about potential B-cell involvement in MS. For instance, one new study suggests that B cells might attack the CNS by secreting cell-killing toxins (Lisak et al., 2012).
Evidence for antibody-independent B cell functions in MS broadens the base of support for B cells’ involvement in the disease and adds to a growing body of research that recognizes myriad mechanisms of immune cell attack. Swiveling the spotlight to B cells could expose what goes wrong in MS and spark new ideas for treating the disease.
Enter B cells
In healthy brains, cells called oligodendrocytes sheathe neurons in insulating layers of myelin. The fatty coating acts as a protective jacket and helps messages dart along cells; this speedy transit is crucial for proper brain function. Oligodendrocytes also release chemicals that help keep nerve cells healthy. Scientists agree that MS damages the external myelin layer and the neuron underneath, but they’re less certain about what triggers the disease’s characteristic demyelination. “We don’t really know what starts MS off,” says Amit Bar-Or, a neurologist at the Montreal Neurological Institute and Hospital at McGill University in Canada (who is an Accelerated Cure Project Scientific Advisory Board member). “It’s still an outstanding question.”
Early insights into myelin damage and attack came from animal models of MS. Experimental autoimmune encephalomyelitis (EAE) mice helped cast T cells as the divas of disease pathogenesis (Kasper and Shoemaker, 2010, and “Animal Arsenal”). Because EAE T cells—and not B cells—can transfer the disease from mouse to mouse, and because T cells crop up in patchy inflammatory zones inside MS patients’ brains, many researchers focused on T cells’ part in the disease. What’s more, the biggest genetic risk factor in MS lies in the MHC region of the genome, which encodes proteins that aid T cell activation. But treatments that affect only T cells have failed in MS trials, says Timothy Vollmer, a neurologist at the University of Colorado, Denver, School of Medicine in Aurora. “Despite the perception that this is a T-cell-driven disease, we do not have any selective T cell therapy that works in MS,” he says. Indeed, every approved treatment for MS blocks some form of B-cell activity (Kitsos et al., 2012). Therefore, Vollmer says, B cells deserve as much scrutiny as T cells do.
A potential role for B cells in MS has been bubbling in the background for decades. In 1960, Armand Lowenthal and colleagues at the Institut Bunge in Antwerp, Belgium, spotted B cells in MS patients’ cerebrospinal fluid (CSF)—the liquid that cushions the brain and spinal cord. When tested in the lab, CSF from these patients tattooed protein gels with “oligoclonal bands”—a hallmark of antibodies and thus a signature of B cells. These bands pop up in the CSF of more than 95% of MS patients (Franciotta et al., 2008) and might come from a ballooning population of antibody-pumping B cells in the brain (Dobson et al., 2011).
In 1979, neurologist John Prineas, then at the College of Medicine and Dentistry of New Jersey-New Jersey Medical School, reported “thin-walled channels” of lymphoid tissue—which is packed with immune cells—in the brains and spinal cords of MS patients (Prineas, 1979). Aloisi and colleagues extended these findings in 2004, when they discovered that B cells were setting up shop in finger-shaped projections inside MS patients’ meninges, the three-layer sandwich of membranes that encases the CNS. Because B cells normally mature and multiply only in lymphatic organs, which reside outside the brain and spinal cord, their presence in the CNS waves a red flag to researchers. “It’s well documented that the B lymphocytes we see in the CNS are undergoing full development there: Everything they’re normally doing in the lymph nodes, they’re doing in the brain,” Vollmer says. “That’s unusual.” What’s more, the “follicle-like structures” overlap with damaging lesions in the cortex, the wrinkled outer covering of the brain. The suspicious structures also harbor other immune players, but because they’re relatively rich in B cells, Bar-Or says, “it’s very attractive to speculate that [B cells] may contribute importantly to inflammation and injury within the CNS.”
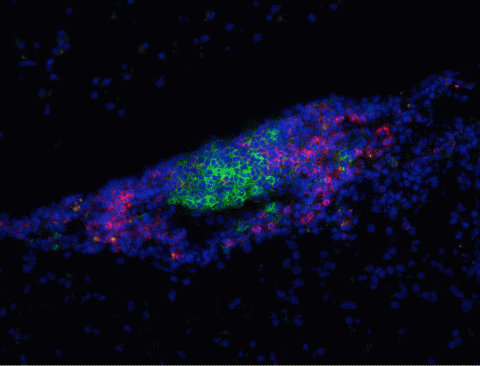
Although B cells in MS might target myelin by making self-reactive antibodies that mark the fatty wrapper for immune attack, researchers have never bagged a definite disease-causing autoantigen. One recent study tested whether antibodies from MS patients could strike myelinated cells in culture. Researchers collected blood serum from MS patients, healthy controls, and patients with other neurological diseases. Then they purified antibodies from the serum and added the molecules to rat neurons in culture. They found that 30% of patients with MS harbored autoantibodies that could damage the myelinated cells; material from healthy subjects and individuals with other neurodegenerative diseases could not.
“The absolute bottom line is that this confirms [that] at least a subset of patients with MS develop autoantibodies that can mediate primary demyelination and axon injury,” says Christopher Linington, the study’s senior author and an immunologist at the University of Glasgow in the U.K. The findings, published 4 May 2012 in Brain, reinforce the idea that autoantibodies contribute to MS onset or progression (Elliott et al., 2012). But because researchers detected the self-reactive molecules in only a third of MS patients, the results suggest that these antibodies are not the central drivers of the disease, says Leila Jackson, a neurologist at the University of Colorado, Denver, School of Medicine in Aurora.
Linington acknowledges that the total percentage of MS patients with myelin-targeting antibodies was lower than he expected, but he says the result might arise from a technical issue. “We’re using rat cultures to detect human antibodies,” he says. “If the antibodies recognize a human antigen but not a rat antigen, there would obviously be patients we’d miss.”
Taking on new roles
Clues about B cells’ antibody-independent roles in MS might reveal themselves when researchers pull back the curtains on rituximab’s mechanism of action. The genetically engineered antibody sticks to a surface protein—CD20—on B cells and kicks off an immune cascade that clears B cells from the blood (Kitsos et al., 2012). Because it doesn’t knock out plasma B cells—the antibody-makers—or knock down antibody levels in the CSF, the drug’s showstopping results cued scientists to look for other aspects of B-cell performance.
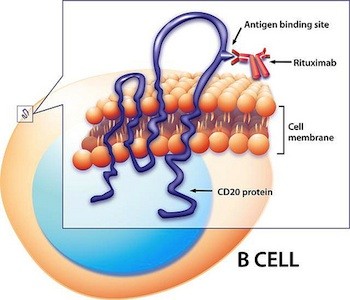
Although rituximab is known for depleting B cells from the blood, it’s conceivable that some of the drug’s clinical benefits stem from its ability to target B cells in the brain or meninges. However, many researchers find this scenario unlikely: Because rituximab is an antibody, and thus bulky, significant quantities couldn’t slip into the CNS unless the blood-brain barrier was seriously broken, they say. What’s more, if B cells tucked away in follicles rev up trouble in MS, it’s even more of a stretch to imagine that rituximab or other B-cell-targeted drugs could reach them. Meningeal follicles are solid structures hundreds or thousands of cells deep, Aloisi says. It’s hard to know how far the drug could penetrate, she says.
Regardless of where rituximab acts, one possible way it works is by interfering with B cells’ influence on T cells. Getting rid of B cells limits the number of T cells in the CNS and curtails exaggerated T cell activation in MS patients, Bar-Or says. For instance, B-cell-depletion therapy snuffs out B cells that produce IL-6, an inflammatory compound that urges T cells to proliferate, according to a report by Tom Barr of the University of Edinburgh and colleagues, including Bar-Or. Their results, published 30 April in The Journal of Experimental Medicine, were from experiments on EAE mice but might also apply to humans (Barr et al., 2012). The authors note that B cells from MS patients secrete more IL-6 than do cells from their healthy counterparts.
Other researchers have suggested that rituximab targets B cells’ antigen-presenting ability. Usually, antigen-presenting B cells decorate their surfaces with bits of bacteria or other invaders to alert T cells about likely villains—thus creating a molecular “most wanted” list. Rogue B cells could adorn themselves with pieces of myelin, directing T cells to attack the body instead of the intruders. Exposing such rebel antigen presenters might help explain rituximab’s effects.
Toxin-brewing villains
As scientists probe how rituximab and other B-cell-depleting therapies—such as mitoxantrone and ofatumumab—work, new research is pointing toward alternative methods by which B cells contribute to MS through activities other than antibody production. They might, for instance, make chemicals that poison brain cells, Bar-Or and Lisak reasoned. Other immune cells “secrete substances that have been shown to damage oligodendrocytes,” Lisak says. “It made me think that B cells might be able to do it too.”
Lisak and Bar-Or’s research teams harvested B cells from the blood of seven patients with relapsing-remitting MS and four healthy controls; then they tested whether cells from each group could spit out brain-damaging toxins. After cultivating the donors’ cells in the lab and adding a slew of different molecules to stimulate B cell activity, they siphoned off the broth in which the cells grew and added it to mixed cultures of rat glial cells, non-neuronal cells of the CNS.
Fluid from patients’ B cells killed significantly more rat cells than did growth media from healthy controls, the researchers reported 28 March in the Journal of Neuroimmunology (Lisak et al., 2012). And the toxic brew seemed to zero in on one particular cell type: oligodendrocytes. Within 3 days, these myelin-makers shriveled and sloughed off the petri dishes in fragmented sheets. Neighboring rat cells in the culture—astrocytes and microglia—escaped injury-free.
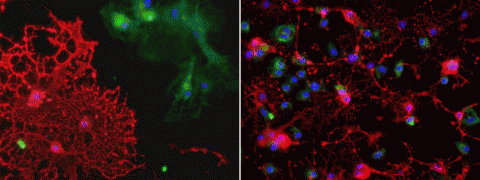
This work is the first to show that B cells have a T cell- and antibody-independent mechanism that leads to oligodendrocyte death, Vollmer says. The finding is important, he says, because people have always assumed “that antibodies or activation of T cells mediate the toxic effect on oligodendrocytes.”
But Aloisi cautions that the results are preliminary: “The subject is interesting, but the sample size was very limited.” Furthermore, using cultures of rat cells could complicate the findings: “You cannot exclude that something can be toxic to rat oligodendrocytes but not to human oligodendrocytes,” she says. However, because human oligodendrocytes come only from patient biopsies, she concedes, it’s very difficult to harvest enough tissue for the types of experiments performed in the study.
Still, the finding “certainly deserves further investigation,” says Gareth John, a neurologist at Mount Sinai School of Medicine in New York City. He wants to know whether the toxic effect on oligodendrocytes is mediated by B cells directly. Because researchers added the B cell medium to a mixture of CNS cells, it’s possible that other cells in the culture—astrocytes and microglia—provoked oligodendrocyte death.
“I think that’s one of the interesting questions that now should be chased down,” Bar-Or says. “What exactly is the mechanism, and what are the molecules that are involved?” The researchers tried to pin down the toxic factor’s identity by screening for the usual suspects—antibodies, cytokines, and growth factors—but netted no culprits. Bar-Or says that the team has a few leads, but “none that would be ripe for naming” just yet.
If toxic B cells do play a leading role, and if researchers could target the rogue actors, it might be possible to selectively deplete the bad ones. Because treatments such as rituximab are relatively safe compared to other MS therapies, Bar-Or says, directing drugs toward even smaller subsets of cells could mean therapies with fewer side effects. Furthermore, John says, understanding what controls oligodendrocyte survival could point scientists toward strategies for protecting the nerve-insulating cells. Doing so “may lead to reduced tissue damage in patients.”
Next acts
As scientists audition novel hypotheses for how B cells might act in MS, they are scouting far and wide for breakout performances. In addition to making autoantibodies, talking to T cells, infiltrating the meninges, and secreting toxins, B cells—or other immune players—might, for instance, erode myelin by attacking neurons, whose back-and-forth chatter with oligodendrocytes is essential for making the lipid-laden sheaths. Injury to neurons, therefore, could act as a muzzle, cutting off communication and subsequently myelin maintenance, says Vittorio Gallo, a neuroscientist at Children’s National Medical Center in Washington, D.C. Rather than operating through one mechanism or another, however, demyelination, Gallo says, “is likely to occur through a mosaic of different scenarios.”
Although B cells aren’t performing solo, they seem to be the new rising stars of MS research. Now that these immune actors have nudged their way closer to center stage, scientists are lining up to observe how B cells’ talents and blunders play out. Examining how they bumble the immune performance will broaden our understanding of the disease and might set the scene for new treatment options.
Key open questions
- By what mechanism does B-cell depletion improve patient outcomes?
- Does rituximab target B cells in follicle-like structures?
- How do follicular B cells colonize the meninges?
- What substance (or substances) mediates MS patient B cells’ toxic effect on oligodendrocytes?
- Is the toxic factor(s) specific to patients with MS, or is it present in patients with other autoimmune diseases?
- Does the toxic factor(s) directly harm oligodendrocytes, or does it stimulate other cells (such as astrocytes or microglia) to produce something that harms oligodendrocytes?
- Can the toxic factor(s) injure neurons as well as oligodendrocytes?
Image credits
Thumbnail on landing page. "State Theater" bagaball/Wikimedia Commons, 2008. Released under a Creative Commons Attribution 2.0 Generic (CC BY 2.0) license.
Fig. 1. Courtesy of Barbara Serafini and Francesca Aloisi, Istituto Superiore di Sanità, Rome, Italy.
Fig. 2. "Rituximab targets B cells by binding specifically to CD20 on the cell surface." Courtesy of National Institute of Allergy and Infectious Diseases.
Fig. 3. Courtesy of Robert Lisak, Wayne State University.



Researchers who study multiple sclerosis have long debated whether MS is a bona fide autoimmune disease in part because a clear, disease-causing autoantigen has never been identified despite extensive efforts to find one. In this study, Elliott et al. used a novel assay for the search: They exposed myelinated rat neurons cultured from spinal tissue to blood serum taken from individuals with MS (n = 37), healthy controls (n = 13), and those with other neurological diseases (n = 10) to test whether antibodies in the serum could attack myelin antigens and cause demyelination. They identified pathogenic immunoglobulin G responses in the serum of approximately a third of the MS group but not in serum from any other subjects. This observation suggests that autoantibodies can contribute to demyelination by targeting oligodendrocytes and the myelin sheaths they extend. Three people from that subgroup who had undergone plasma exchange therapy showed significantly reduced demyelinating IgG activity after the procedure. Further work is necessary to determine the relation between the antibodies, such treatment, and disease course.