Tracking Remyelination: Do You See What I See?
Several new imaging modalities are aimed at evaluating possible remyelination in patients with multiple sclerosis. This article discusses how the techniques work and their potential applications to MS, as well as their limitations.
For nearly 15 years, magnetic resonance imaging (MRI) has been an important tool for diagnosing and managing patients with multiple sclerosis.
But conventional MRI techniques—such as T2-weighted imaging, T1-weighted imaging, and post-gadolinium T1 weighted imaging—have significant limitations.
One is a lack of specificity: an inability to distinguish among various types of pathologies such as edema, ischemia, cell loss, gliosis, demyelination, and inflammation. Another is that they are unable to accurately assess the extent of demyelination injury during and after exacerbations. Still another is their inability to sort out all of the different physiologic abnormalities associated with MS.
For example, significant white matter abnormalities often appear quite normal on conventional scans. And even though demyelination of the spinal cord is a common feature in patients with MS, conventional imaging often fails to adequately pick up those changes.
The need to see more
Over the past few years, researchers have been working with various new compounds aimed at not only holding MS at bay but also reversing demyelinating damage wrought by the disease. To that end, several trials are getting underway to evaluate those treatments.
"For such trials to be successful in determining whether or not there is a real effect from these interventions, we need appropriate outcome measures," Shahrukh Mallik, MRCP, Institute of Neurology, University College London, told MSDF in an email correspondence. Mallik, who recently co-authored a paper reviewing new imaging techniques (Mallik et al., 2014), went on to write, "Clinical outcomes are, of course, important, but we would also like to detect effects on myelin in vivo. We thus need sensitive imaging measures that are reliable, reproducible, and realistic for use in clinical trials."
Robert J. Fox, M.D., of the Mellen Center for Multiple Sclerosis at the Cleveland Clinic Foundation in Ohio, agrees. In a telephone interview with MSDF, he emphasized that although conventional MRI modalities are a great tool for assessing MS, their use is limited. (Dr. Fox is not related to Steven Fox, the author of this article.)
For instance, he said, those techniques aren't very useful in the later stages of MS when inflammation is not as prevalent as in earlier stages. "What's going on instead is a degenerative process, or some sort of chronic decline that is somewhat separate and distinct from infiltrative inflammation. So, as a result, we need to look to other imaging modalities to gain insight into what is happening." He said the hope is that with emerging advanced MRI techniques, clinicians and researchers will be able to closely monitor the effects of new drugs on myelin in the brain, spine, and optic nerve.
Brain atrophy studies
One of the imaging techniques for MS that's been used extensively over the past decade centers on measuring brain atrophy. Inflammation in MS is linked to demyelination as well as to axonal loss. The result can be tissue loss, and such changes can be spotted with brain atrophy studies.
Brain atrophy is seen throughout the course of MS, and even though it's not necessarily associated with clinical measures of disability, it has been shown to predict later-stage progression of disability. Paradoxically, the anti-inflammatory effects of some MS drugs can actually reduce brain volume.
"Advanced MRI imaging techniques for measuring brain atrophy can be a good metric especially for trials focusing on use of experimental neuroprotective agents, where conventional lesion measures don't do a good job of characterizing the underlying neurodegeneration," Fox said.
Still, brain atrophy measurements don't provide all the answers. One problem is that they generate only one measurement per study, in other words, a measurement of the volume of the brain at a single point in time. In addition, variations in patient hydration can skew measurements of brain volume. "That's significant, because it adds a lot of noise to an outcome measurement that you're hoping is measuring your disease, but it's also measuring other banal things like hydration," Fox said.
Magnetic transfer ratio
In an effort to get around those limitations, researchers have developed several newer MRI techniques. One is measurement of magnetic transfer ratio (MTR).
Most commercial scanners can already acquire MTR images, Mallik explained in his paper.
MTR is based on the premise that protons that are part of biological tissue exist in two pools. One is a free (“liquid”) pool, in which protons are highly mobile; the other is a restricted (“semisolid”) pool that is made up of protons linked to macromolecules such as proteins or lipids, which are therefore relatively immobile, Mallik wrote.
MTR uses two scans per study—one with and one without MT-weighting—to quantify transfer of magnetization from tissue structures to surrounding water molecules. Sites of tissue injury and tissue destruction show up as decreases in transfer magnetization from tissue infrastructure to adjacent areas. MTR studies are significantly specific to detect myelin but can also be affected by water content as well as inflammation, Mallik wrote.
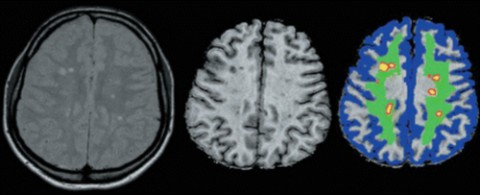
Another problem affecting MTR and, for that matter, other types of MR imaging is patient movement during the scanning session. When patients move during scans, acquisition of high-quality images is difficult at best. The long length of most scanning sessions is at the root of the problem. Researchers are trying to come up with refinements in hardware and software that will allow for shorter scan times.
Even so, images obtained with MTR are thought to provide a good representation of myelin status, although no doubt other factors aside from the presence or absence of myelin or patient movement during scans can affect magnetization transfer ratios.
In a telephone interview, Bruce Cree, M.D., of the University of California, San Francisco, told MSDF there's been considerable speculation that MTR may be a marker for remyelination, but it hasn't yet been found by histopathological correlates to definitively represent that process.
"And that's sort of the problem that the field has right now," he said. "When you're looking at lesions as they evolve over time and gradually get better, there are many things that go on within the lesion that result in particular imaging signals."
Edema is one of those problematic phenomena. "When we have an acute lesion, there can be a lot of water present," Cree explained. That, in turn, produces a deflection in the MT ratio that resolves as edema subsides. "So the key question that nobody knows the answer to," he said, “is, when you're looking at MTR in an acute lesion, are you really looking at a remyelinating process, or are you looking at the resolution of tissue edema, or some combination of these things? And that, I think, is the big limitation in the field right now.
Diffusion tensor imaging
Diffusion tensor imaging (DTI) is another advanced MRI modality that shows promise in being able to detect subtle changes that may signal neuroprotection or remyelination.
DTI uses 3D imaging to assess the ability of water molecules to move along nerve fibers. "When you've got large structures—these axonal structures that are forcing water molecules to only move along one axis—you can use diffusion tensor imaging to examine that process," Cree explained. "You can provide precise values about the constraint of water molecules, that is, how much freedom they have to move in a three-dimensional space."
DTI picks up abnormalities in white matter that may appear normal using conventional scans. It can also discriminate among different types of lesions. In addition, DTI has been shown to correlate with several clinical, physiologic, and psychological scores, and thus may prove useful in evaluating disease progression and response to therapy in individual patients.
But DTI has its shortcomings. For example, although DTI is a good method for imaging highly organized tracts of white matter, it is less effective in providing accurate quantifiable results in more complex tissues, such as crossing fiber tracts, gray matter, and areas of tissue injury. Researchers are busy trying to come up with workarounds for those limitations.
Even with their limitations, however, DTI and MTR are the two most viable options for use in multicenter trials of patients with MS. Manageable scan times and specificity to myelin add to their appeal.
"The thing that's special about these two techniques is that they're quantitative, so you can get an actual number out of the sequence, and they both relate back to tissue integrity," said Robert Naismith, M.D., of Washington University in St. Louis, Missouri. Naismith, who spoke with MSDF on the telephone, added, "They tell you how much destruction is taking place within the tissue, whereas the conventional methods don't give you that information. They only tell you that something's abnormal, but they don't tell you how abnormal it is. So I think each of these techniques can provide information to help support whether a medicine can have an overall effect on tissue integrity."
MR spectroscopy
Magnetic resonance spectroscopy (MRS) is another potential modality for measuring neurodegeneration, brain stabilization, and remyelination.
Whereas conventional MRI characterizes the physical properties of targeted tissue, MRS characterizes chemical properties.
It's been used to assess changes and imbalances that occur over the course of MS, usually measuring various cellular metabolites, such as N-acetylaspartate, creatine, choline, glutamate, glutamine, and myoinositol.
With the ability to quantify such a wide range of metabolites, MRS could turn out to be a useful tool for assessing the efficacy of MS therapies, including those that are focused on remyelination, researchers say.
"The tricky part about spectroscopy, however, is the variability that's seen with spectroscopy measurements," Fox said. "That may make it tough to implement MRS in a longitudinal multicenter trial."
So far, only a few studies have been done using MRS to gauge the effects of MS therapies, Fox said, and the results have been mixed. Still, researchers continue to hone what may be an important tool.
Functional MRI
Functional MRI (fMRI) is yet another technique investigators are using to better understand the effects of MS and develop appropriate therapies. fMRI assesses how variations in brain activity affect MRI signals.
These types of studies most often involve having the patient perform a specialized task during the scan session. The patient's performance is correlated to MRI signals to establish various parameters relating to neuronal activity.
Tasks can be specially geared toward stimulating various parts of the brain, such as those that handle cognitive, emotional, motor, or sensory tasks. Study designs most often compare MS patients with controls or with different subgroups included under the MS diagnosis.
Most of the fMRI studies done so far have looked at compensatory processes and reorganization of functional tissue. The results suggest that compensatory processes that take place in the brain tend to skew the way neuropathology in MS corresponds to perceived disability status.
New studies underway
Several new studies are in the works comparing the performance of some of the new MRI techniques.
Fox is helping run one of the trials. It's a phase 2 multicenter study known as the SPRINT-MS trial), and its aim is to use several imaging techniques—MTR, DTI, and brain atrophy studies—to assess the effects of ibudilast, one of several compounds that researchers are hoping will prove to be neuroprotective and possibly even spur remyelination.
"This is not a substudy; it's an across-the-whole study," Fox said. "So at the end not only will we find out if the drug we're evaluating appears to be protective, but we will also be able to compare the imaging metrics side-by-side to evaluate which one is the most robust and reliable to use in a multicenter trial."
That's just one of a number of potentially promising lines of remyelination research that investigators are mining.
Enthusiasm and skepticism
Although enthusiasm for new imaging modalities aimed at spotting remyelination is considerable, a fair degree of skepticism remains regarding their usefulness, at least at their present stage of readiness.
"The question of how best to image myelin in vivo—and, in particular, demyelination and remyelination—is, in my view, still very much open," Daniel Reich, M.D., Ph.D., said in an email correspondence with MSDF. Reich, of the U.S. National Institute of Neurological Disorders and Stroke, said of the existing techniques, "In a general sense, I don't really like any."
He added, "In fact, I would say that there is no current technique that can selectively and specifically image myelin with the fidelity that would be required to prove that demyelination or remyelination occurred. If that's the question that needs to be answered, in vivo human imaging isn't the way to do it."
Not a panacea, but a valuable tool
Advanced MRI, then, may not be a panacea, but it could prove to be a valuable tool, in a variety of respects, for managing patients with MS.
To understand how the disease manifests in patients, researchers need to consider a wide range of factors besides MRI findings that may influence disease progression and outcomes.
"We're really in the infancy of discovering the various therapies in MS and other neurologic disorders," Naismith said. "I take the approach that in this early stage we need to be open-minded and look at all of the techniques in terms of how they can inform us about the way a particular therapy is working."
He added, "Right now we're blazing a new path in terms of what this might look like in the next several years. As an investigator, I find this to be quite an exciting time, because nobody knows for sure exactly how it will play out."
Key open questions
- If remyelination is seen on advanced MRI studies, how will histopathological findings correlate with those changes?
- As researchers and clinicians gain expertise in the use of advanced MRI studies, what clinical value will radiologic endpoints provide?
- What will be the best clinical outcomes measure for assessing remyelination?
- Currently available evidence suggests that the dynamic changes seen on MTR may correlate with the extent of myelination and remyelination. Will this be confirmed in future studies?
- What relationship will future studies find between the presence of lesions in the spinal cord and abnormal findings on MTR?
- When the subject of an MRI study moves during a scanning session, acquisition of high-quality images is compromised. Part of the problem is the extended length of some scanning sessions. How successful will research efforts be in devising refinements in hardware and software that allow for shorter scan times?
Disclosures and sources of funding
Dr. Cree reports having received personal compensation for consulting from AbbVie, Biogen Idec, EMD Serono, Genzyme/Sanofi Aventis, MedImmune, Novartis, and Teva Neurosciences, and has received contract research support from Acorda, Avanir, Biogen Idec, EMD Serono, Hoffmann La Roche, and Novartis.
Dr. Fox has reported receiving financial compensation from Biogen Idec, Novartis, Teva Neuroscience, and Xenoport.
Dr. Naismith reports having received financial compensation from Acorda, Bayer, Biogen, EMD Serono, Genentech, Genzyme, Novartis, and Questcor.
Dr. Reich and Dr. Mallik declared no relevant financial relationships.


