PodBlog: B-Cell Double Header
In episodes 56 and 57 of the MS Discovery podcast, Gavin Giovannoni, MBBCH, PhD, and Timothy Vollmer, MD, discuss the first large-scale study of a drug showing some benefits in a progressive form of multiple sclerosis. The story goes back decades, as Stephen Hauser, MD, recently explained.
A former beauty queen made a big impression on Stephen Hauser, MD, when he was a neurology resident in Boston. Like him, she was age 27. She was an athlete, a Harvard law school graduate, and she worked in the Carter White House. She came from a buttoned-up family and had conducted herself magnificently—until about 6 weeks earlier.
She had become chatty and seductive. She made bad judgment calls at work. She was sent to a psychiatrist, who could not pinpoint the cause. When other signs added up to multiple sclerosis (MS), she was flown to Boston for treatment. She soon lost her ability to speak, swallow, and breathe on her own.
“This case was about the most aggressive MS presentation that even the gray hairs had seen,” Hauser told a roomful of young MS researchers and physicians at the Tykeson Fellows Conference of the National MS Society in Fort Worth, Texas, earlier this month. “I remember thinking that this was the most unfair thing I had ever seen.”
The year was 1977, and there were no specific treatments. MRI, now a diagnostic standard, was not even a medical rumor. Diagnosis included a soak in a warm bathtub, with transient worsening of symptoms likely indicating MS. She was treated with steroids and plasma exchange, neither of which worked out well. The last time Hauser saw her, a year later, was across the street in the rehabilitation hospital. Balloons and flowers decorated a dreary patient room. Her boyfriend had stuck with her. She and her family had organized a wedding.
About the same time, he had another memorable experience. A postdoctoral fellow from the Massachusetts Institute of Technology was giving a seminar in a conference room on the ninth floor of an old building at Massachusetts General Hospital (Hauser, 2015). The neurology chair was openly skeptical that MS was modeled by experimental autoimmune encephalomyelitis (EAE) in rodents, the basis of the talk.
Hauser began a lifelong pursuit of better ways to model MS in the laboratory and dissect the underlying immunology of MS. Results presented at the October 2015 European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) meeting in Barcelona represented a culmination of much work that had unexpectedly implicated B cells and showcased them as a therapeutic target.
Hauser took the stage at ECTRIMS to walk through the preliminary results of B cell-depleting therapy for relapsing-remitting MS. Two large phase 3 studies, OPERA I and II, showed the overall effectiveness of ocrelizumab, which targets the CD20 receptors on circulating B cells.
The next day, the more modest effects of ocrelizumab in people with primary progressive MS made an even bigger splash. The phase 3 ORATORIO trial was the first large study to show benefits of a treatment for a progressive form of the disease.
Several spokespeople for drugmaker Genentech have said the company plans to submit data for regulatory approval in early 2016.

In episodes 56 and 57 of the MS Discovery podcast, Gavin Giovannoni, MBBCH, PhD, a neurologist at Barts and the London School of Medicine and Dentistry in London, and Timothy Vollmer, MD, a neuroimmunologist at the University of Colorado, Denver, School of Medicine in Aurora, discuss the fresh ocrelizumab findings for progressive MS. MSDF talked with them at the ECTRIMS meeting. Listen to the podcasts here or on your iTunes podcast feed.
In summing up the ORATORIO results at ECTRIMS, presenter Xavier Montalban, MD, PhD, of Vall d'Hebron University Hospital in Barcelona, Spain, reported a 24 percent reduction in the risk of sustained disability progression at 24 weeks. A slide showing a 3.4 percent decrease in T2 lesion volume, an MRI brain measure, compared to an increase in the placebo group, drew a hushed whispering from the crowd.
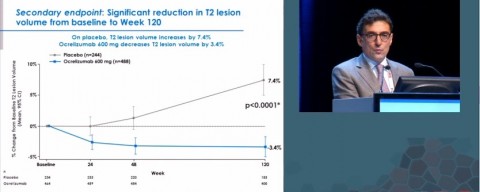
The 24 percent reduction in disability progression cannot easily be translated into a number that is meaningful for an individual patient at this early stage of analysis, said Peter Chin, MD, principal medical director at Genentech, in a recent follow-up phone call with MSDF. It is a statistic arising from a study design counting disability progression events and not a mean or average figure.
As you’ll hear on the podcasts, the main question everyone seems to want to know is what subgroup of the original PPMS population, if any, responds to ocrelizumab. The initial study design specified a younger group of patients more likely to respond. On the phone, Chin stuck to the party line. “I appreciate the excitement,” he told MSDF. “We are in the phase of analyzing the data. ECTRIMS was really fresh. I don’t have anything that wasn’t reported at ECTRIMS.”
Chin promises further analyses will be presented at upcoming scientific meetings to be named later, and fuller details in a peer-reviewed publication at some future time. “Scientifically speaking, it confirms B cells are important,” Chin allowed, especially circulating B cells that express the CD20 antigen. The drug spares progenitor B cells, allowing them to reconstitute the humoral immune system, as well as memory B cells that maintain antibody immunity to infections and vaccinations, such as the annual flu shot.
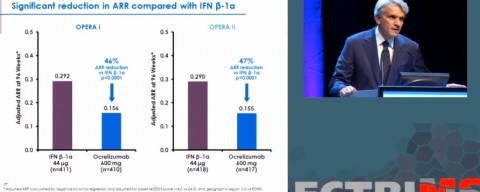
Meanwhile, at the Tykeson talk in Texas, Hauser listed two other earlier “gadzooks” moments in earlier stages of the unfolding anti-CD20 B cell story.
- After much trial and error, a researcher in Hauser’s lab figured out a way to immunize a marmoset, a small monkey about the size of a guinea pig, so that it developed lesions with concentrated demyelination, as in people, rather than the sparse demyelination of mice. The team used a concoction of antibodies against myelin and concluded that the lesions may have arisen from B cells.
- It was a sunny October Friday afternoon when Hauser and his colleagues unblinded a short phase 2 data of rituximab, the first monoclonal anti-CD20 antibody to be tested in RRMS (Hauser et al., 2008). “It was thrilling. We thought we had something useful for patients,” he said. “It was equally thrilling, because the effect was never due to antibodies [as they had believed]. It told us our fundamental hypothesis driving this was wrong. Going from bench to bedside now sent us back to the bench.”
Not all B cell-depleting approaches will be effective in MS, Hauser cautioned. Some may worsen the disease by eliminating regulatory B cells.
Looking ahead to the next generation of B cell-targeted therapeutics, “the phase 3 ocrelizumab trials set the stage for more selective approaches,” he said. “To conclude, the trials taught us in a way that we never could have imagined that B cells are the center of the game in the relapsing component,” he told his Texas audience.
Disclosures and sources of funding
Giovannoni, a member of the MSDF Scientific Advisory Board, was an investigator and trial steering committee member for the ORATORIO trial. Other sources of funding include AbbVie, Bayer-Schering Healthcare, Biogen, Canbex, Eisai, Elan, Fiveprime, Genzyme, Genentech, GSK, GW Pharma, Ironwood, Merck Serono, Novartis, Pfizer, Roche, Sanofi-Aventis, Synthon BV, Teva, UCB Pharma, Vertex Pharmaceuticals.
Vollmer has received compensation from AbbVie, DeltaQuest, Genentech, Novartis, Novartis Canada, Oxford Pharmagenesis, Hoffmann-La Roche, Teva Neuroscience, EMD Serono, WebMD/Medscape, Rocky Mountain MS Center. Vollmer has received research funding from Avanir, Genzyme, Ono, Biogen, Teva, NIH/NINDS, Janssen Research & Development, MedImmune, Acorda, Rocky Mountain MS Center, EMD Serono.
Hauser is supported by the U.S. National Institutes of Health and National Multiple Sclerosis Society.


