Data Visualization Displays 142 Ongoing MS Clinical Trials
Our latest data visualization allows you to sort the trials 10 different ways, including by phase, by population, by compound, and by sponsor
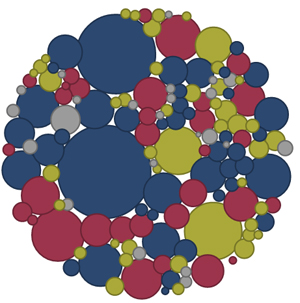 The image to the right comes from our latest data visualization, constructed by the good folks at Khawai. It illustrates, as best as we can determine, every ongoing MS clinical trial worldwide. The size of each bubble corresponds to the targeted sample size of the trial, the color indicates whether the agent being tested is already being marketed for MS (blue), is already being marketed but not for MS (yellow), is not yet being marketed (red), or is in some other category (gray).
The image to the right comes from our latest data visualization, constructed by the good folks at Khawai. It illustrates, as best as we can determine, every ongoing MS clinical trial worldwide. The size of each bubble corresponds to the targeted sample size of the trial, the color indicates whether the agent being tested is already being marketed for MS (blue), is already being marketed but not for MS (yellow), is not yet being marketed (red), or is in some other category (gray).
In all, we’re displaying data on 142 ongoing clinical trials with a targeted total sample size of 55,758 patients.
If you visit the visualization, you’ll see that mousing over a bubble reveals many other details about the trial. Best of all, you can display the trials in 10 different ways by clicking the tabs at the top and bottom of the image. You may display all trials, as in the image above, or you may sort by:
- Phase
- Population
- Compound
- Number of arms
- Sponsor
And by the interaction of
- Compounds and sponsors
- Phases and compounds
- Populations and sponsors
- Arms and sponsors
One of the beauties of a data visualization is that it allows you to see interesting relationships you might not notice by looking at the data in a spreadsheet (although if you want to manipulate the data yourself, we’ve made it possible for you to download a comma-delimited CSV file; click on Data Source at the bottom of the page).
One thing I noticed right away when I sorted by the interaction of compounds and sponsors is that the largest ongoing trials are sponsored by large pharmaceutical companies (not that surprising) and mostly involve compounds already marketed for MS. I think I would have expected the largest trials to be compounds that were not yet being used for MS.
Please do visit our data visualization, and please share any interesting finds in the comments section here.


