Phase 2 Trial Suggests RPC1063 Is as Effective as—and Safer Than—Fingolimod
RPC1063, an experimental oral immunomodulator, is looking promising as it progresses through clinical testing. At the ACTRIMS-ECTRIMS meeting in September, Amit Bar-Or presented results of the phase 2 trial suggesting the drug is as effective as and safer than its counterpart, fingolimod.
RPC1063 may open the door to more convenient treatment options for relapsing-remitting MS, if recently released phase 2 results are any indication. On the last day of the joint ACTRIMS-ECTRIMS meeting in September, Amit Bar-Or, M.D., FRCPC, of McGill University, presented the results in a late-breaking news session (Cohen et al., 2014). The drug proved to be highly effective, and—perhaps more significantly—had fewer side effects than its counterpart, fingolimod.
The goal of the phase 2 trial—also known as the RADIANCE trial, sponsored by Receptos Inc.—was to test the efficacy and safety of the orally administered agent. Investigators randomly assigned 258 RRMS patients to a placebo group, a group taking 0.5 mg of RPC1063, or a group taking 1.0 mg of the drug. Before anyone was given a full dose of the drug, they underwent a titration period of 7 days. The treatment period continued for 24 weeks, followed by a yearlong safety extension period during which everyone took either 0.5 or 1.0 mg of the drug.
At the end of the initial 24-week treatment period, patients in both groups taking RPC1063 showed an 86% decrease in the cumulative number of gadolinium-enhanced lesions compared to the placebo group. The annualized relapse rates also dropped in the treatment groups when compared with placebo, with a 31% decrease in the 0.5-mg group (p = 0.271) and a 53% decrease in the 1-mg group (p = 0.053).
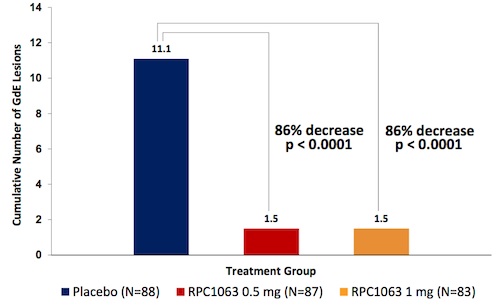
Bar-Or said that these effects are on par with those of fingolimod, the only other oral agent approved for the treatment of MS. Indeed, both drugs modulate the immune system via the sphingosine-1-phosphate receptor family. But the benefit of RPC1063 is considered to be “the ability of the drug to achieve the same degree of impact on the disease with a more palatable safety profile,” Bar-Or said.
Fingolimod affects several organ systems, Bar-Or explained. When patients first begin the treatment, they must be monitored for several hours in case any cardiovascular problems arise. RPC1063, on the other hand, doesn’t seem to induce similar cardiovascular effects, Bar-Or said. He said that so far the drug is looking “boring in a good way.”
The most common side effects that trial participants reported were nasopharyngitis, headache, and urinary tract infections. The researchers also noted an increase in liver enzymes in some participants, but these did not come with any symptoms and did not cause concern.
Of course, the phase 2 sample size was relatively small, and Bar-Or remained level-headed about the prospects of RPC1063. “If [the phase 2 results are] recapitulated in the phase 3 program, then this will look exciting,” he said.
Key open questions
- How will RPC1063 and fingolimod compete against each other in the market?
- What is the best way for doctors to come to a decision with their patients on the best way to treat multiple sclerosis?
Disclosures and sources of funding
Amit Bar-Or receives financial support from Biogen Idec, DioGenix, Genentech, GlaxoSmithKline, Guthy-Jackson Charitable Foundation, Medimmune, Merck Serono, Novartis, Ono Pharmaceuticals, Receptos, Roche, Sanofi-Aventis, and Teva Neuroscience. Bar-Or is a scientific advisory board member of the Accelerated Cure Project, the parent organization of MSDF.



Comments
The results of the Phase II RADIANCE trial offer an expanding horizon for the current disease modifying therapy landscape. The findings of significant reductions in gadolinium-enhancing lesions on MRI, new T2 lesions on MRI, and annualized relapse rate in both dosage arms of the RPC1063-treated group are promising for the development of yet another highly effective oral agent for the treatment of RRMS. The safety data are particularly interesting as RPC1063 has a similar mechanism of action to Gilenya, however RPC1063 acts on less sphingosine-1-phosphate receptor subunits than Gilenya. As RPC1063 was found to have no notable cardiac, pulmonary, ophthalmologic, infectious or malignancy adverse events, it is tempting to attribute the positive outcomes to its more selective mechanism of action. The robust efficacy findings and favorable safety outcomes make RPC1063 an attractive oral agent and it will be interesting to follow the phase 3 data.