Evaluating Patient Preferences Is Key for MS Decision-Making
Taking patient preferences into account may significantly affect patients’ initial and long-term compliance with therapy
Evaluating patient preferences is a crucial step in shared decision-making in relapsing–remitting multiple sclerosis (RRMS), and it’s one that may significantly affect patients’ initial and long-term compliance with disease-modifying therapies (DMTs), according to a study published in June in the Journal of the Neurological Sciences (Wilson et al., 2014). The conjoint analysis study looked at the tradeoff between risks and benefits for preference-sensitive treatment choices important to decision-making regarding DMTs.
“The complexity of choices may lead to patient uncertainty and confusion resulting in barriers to treatment initiation and adherence,” wrote Leslie Wilson, Health Policy and Economics, Departments of Medicine and Pharmacy, University of California, San Francisco, and colleagues. “Physicians are moving away from a model of making treatment decisions for the patient to a shared decision-making model, which necessitates reviewing patient preferences. Shared decision-making models promote better outcomes for patients and are especially important for RRMS patients for which multiple reasonable treatment options exist, with none clearly outperforming the others.”
Specific goals of this study were to calculate relative patient preferences for current risk and benefit attributes of hypothetical DMTs and to quantify patient willingness to accept DMT-associated risks for benefits gained.
Between 2012 and 2013, 289 patients with RRMS completed a choice-based conjoint analysis (CBC) survey. Mean age was 42.0 ± 10.24 years; 76% were female; 78% were Caucasian.
To model the decision-making process and tradeoffs of patients choosing DMTs, the investigators based the survey on all possible DMT attributes. Mixed-effects logistic regression allowed analysis of patient preferences and estimation of maximum acceptable risk tradeoffs for various DMT benefits.
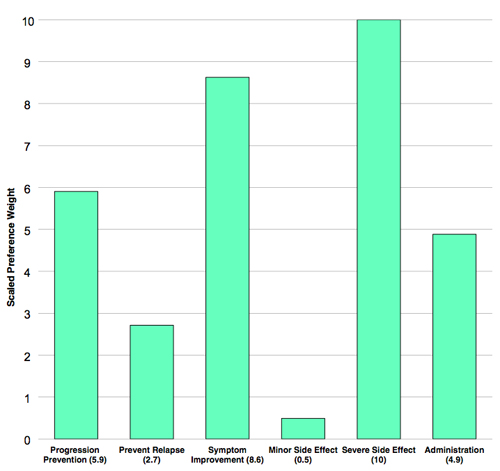
Risk of severe side effects plays largest role
The largest effect on patient preference was risk of severe side effects. A 1% risk reduced patient preference fivefold compared with no risk (odds ratio [OR], 0.22; P < 0.001). As the risk of severe disability or death increased, patients preferred the treatment less, with a linear relationship. However, persons with RRMS were willing to accept increasing amounts of this type of risk in exchange for increasing gains in certain benefits of DMTs.
Minor side effects—including mood changes, headache, muscle or joint aches, flu, and increased risk of infection—had no statistically significant effect on patient preference.
The most preferred benefit was symptom improvement (OR, 3.68, P < 0.001), followed by prevention of progression at 10 years (OR, 2.4, P < 0.001), and then by daily oral administration (OR, 2.08, P < 0.001).
“The high desire for symptom improvement shown here, which is not well satisfied by current treatment, may affect patients' desire to initiate treatment with DMTs and remain adherent to it,” the study authors wrote. “Additionally, the importance patients demonstrated for symptom improvement acts as a reminder to health care providers to focus on symptomatic management of MS along with the discussions of DMT treatment choice.”
For a delay in relapse of 1 year, patients were willing to accept 0.08% severe risk (death or disability). For prevention of progression for 4 versus 2 years, they were willing to accept 0.22% severe risk.
Persons with RRMS strongly preferred oral medications over other routes of administration and were willing to accept more than half a percent risk of severe side effects for this benefit, even if they had to take oral medications daily. They also preferred a monthly intravenous infusion over other more frequent self-injections, but the investigators could not determine whether this preference was driven by the monthly frequency or by not having to self-inject.
Study limitations include possible lack of generalizability to other sites of practice including community or general family medicine practices and reliance on self-reported data regarding treatment histories and relapses.
Improved decision-making
The investigators noted that some study patients and physicians reported feeling more equipped to make a DMT treatment decision after they completed the CBC survey. Plans are underway to develop this survey into a decision-making tool for persons with RRMS who need to decide when to initiate DMT, and with which drug.
“Finally, regulatory bodies also may find our information about risk–benefit trade-offs valuable in their determinations of whether new drug benefits are worth the risks,” the study authors concluded. “These decisions require evaluation of the perspectives of society (through their regulatory bodies), treating physicians, and also the relevant patients. This study will provide the patient perspective.”
Key open questions
- How would routine use of a CBC survey regarding DMTs affect patient and physician decision-making regarding choice of DMT?
- How might these findings regarding patient preferences best be incorporated into pertinent study endpoints in clinical trials and in new drug development?
Disclosures and sources of funding
Leslie Wilson was funded by Novartis Corporation for conduct of this study and has a family member who works for Biogen Idec Inc.



Comments
This study is one of the first to look systematically at patient preferences and risk tolerance in selection of MS disease-modifying therapies (DMTs) using the “choice based conjoint” methodology, which presents random pairs of hypothetical MS DMTs with differing levels of benefit and risk and forces a choice between the two. The use of hypothetical DMTs reduces the likelihood of bias from previous patient experience with these agents. Important conclusions include that MS patients are generally willing to accept a higher level of risk (up to a 1% risk of serious adverse effect or death) if there are important clinical benefits from the DMT. Symptom improvement was most important, followed by delayed disability progression, and delay to next relapse. Since symptom improvement is not typically measured as an outcome measure in MS clinical trials, there is an important gap between patient priorities and accepted MS outcome measures. Not surprisingly, patients preferred oral administration over other routes of administration and were willing to accept a 0.59% risk of serious adverse events just to be on an oral medication. The results of this study provide preliminary data on how patients process risk versus benefit in their decision-making, inform MS clinicians about what patients feel is important, and challenge MS clinical trialists to include new outcome measures in clinical trials that are more relevant to patients.