Nasal Vaccine May Be Effective for SPMS
A study in a mouse model of secondary progressive multiple sclerosis suggests that a nasal vaccine that regulates innate immunity may halt clinical disease and neurodegeneration
Most multiple sclerosis patients are initially diagnosed with the relapsing-remitting form of the disease (RRMS), but over time many will convert to secondary progressive MS (SPMS). While nine drugs have been approved in the U.S. for treating RRMS, none have been shown effective in treating SPMS. In an interview with MSDF, Lior Mayo, Ph.D., of Brigham and Women’s Hospital and Harvard Medical School said, “Although much progress has been made in the study of RRMS, we still don’t understand the basic mechanisms that drive SPMS.”
Mayo works in the laboratory of Howard Weiner, M.D., who published a paper in 2009 suggesting that neurodegeneration in SPMS is triggered by inflammation and driven by the innate immune system (Weiner, 2009). Weiner’s work showed that in the RRMS phase of the disease, the adaptive immune system drives the acute inflammatory events that produce lesions on MRI scans and cause clinical symptoms, but a shift to innate immunity results in conversion to SPMS.
Now, in their paper presented at the American Academy of Neurology annual meeting in Philadelphia on April 29, 2014, Mayo and Weiner have shown that nasal delivery of a monoclonal antibody targeting CD3 on the surface of T cells effectively treats a model of SPMS by inducing regulatory T cells (Tregs) that regulate innate immunity in the central nervous system (CNS). Unlike anti-CD3 given intravenously, which would deplete T cells, nasal delivery of the antibody stimulates mucosal immunity and the induction of Tregs, Mayo said.
In an SPMS mouse model—nonobese diabetic (NOD) mice that are immunized with myelin oligodendrocyte glycoprotein, resulting in a disease course similar to human SPMS—daily nasal delivery of the anti-CD3 antibody during the progressive phase of the disease halted clinical progression and the progression of neurodegeneration. Nasal delivery of anti-CD3 antibody also induced the production of a Tr1 type of regulatory T cell that secretes IL-10. Blocking IL-10 receptor signaling abolished the beneficial effects, indicating that the anti-CD3 antibody works through the IL-10 pathway.
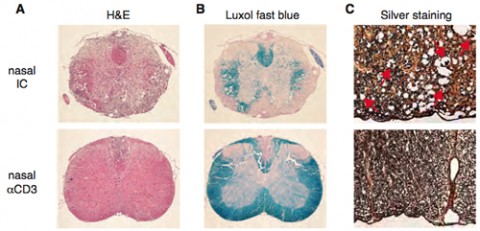
Exploring the mechanisms through which nasally induced Tr1 halted disease progression, Mayo and colleagues found multiple effects, notably reduced peripheral immune responses, attenuated astrocyte activation, and reduced recruitment of inflammatory monocytes to the CNS.
Astrocytes are the most abundant cell type in the brain, and their activation appears to be a central feature of progressive MS, but they’re difficult to study during disease progression. Mayo and his team developed a method to isolate astrocytes and study them at the transcript (mRNA) level, showing that treatment of mice with anti-CD3 antibody compared to a nonspecific control antibody results in numerous changes in astrocytic gene expression, including increases in several genes that are neuroprotective and a decrease in other genes associated with monocyte recruitment and demyelination.
Mayo told MSDF that the team hopes to “to move it from bench to bedside” and to test a humanized form of the nasal anti-CD3 vaccine in humans soon. "This study may allow us to develop a novel approach to treat progressive disease, and it enabled us to gain new insights into the mechanisms underlying disease progression, especially into the role of astrocytes in progressive disease."
Key open questions
- What are the characteristics of the cells induced by the nasally administered anti-CD3 antibody?
- What are the molecular mechanisms by which these cells attenuate astrocyte activation?
Disclosures and sources of funding
Dr. Mayo reported no relevant disclosures. Most of the other authors of the study had nothing to disclose, but F. J. Quintana has received personal compensation for activities with EMD Serono and Teva Neuroscience and research support from EMD Serono. Dr. Weiner has received personal compensation for activities with Biogen Idec, Novartis, EMD Serono, Teva Neuroscience, GlaxoSmithKline Inc., NasVax, Xenoport Inc., and Genzyme Corp., and research support from Merck Serono.
The study was funded by an NIH grant to Weiner and a postdoctoral fellowship to Mayo from the National Multiple Sclerosis Society.



Comments