Myelin and Axons Don’t Form a Perfect, Uniform Union
New findings from mice may rewrite our understanding of just about everything myelin-related. Outside experts describe the study as “landmark,” “novel,” “unexpected,” “new,” “important,” “surprising,” “stunning,” and “provocative.”
Myelin, the fatty insulation that boosts nerve signal conduction, might not be the ubiquitous axonal sheath that the textbooks describe but instead an intermittent and finely regulating conductor of the neural symphony. Giulio Tomassy, Ph.D., and colleagues at Harvard, the Massachusetts Institute of Technology, and the Neuroscience Institute of Turin report today in Science that myelin occurs in definable patterns along axons of specific neuronal groups, including a signature of intermittent myelination punctuated by long gaps of naked axon (Tomassy et al., 2014). Their findings may rewrite the neurobiological understanding of, well, just about everything myelin related, including multiple sclerosis.
Maybe that reads like an overstatement, but all four researchers not involved in the work whom MSDF contacted about this study used words like “landmark,” “novel,” “unexpected,” “new,” “important,” “surprising,” “stunning,” and “provocative” to describe it.
Moses Rodriguez, M.D., a neurologist and MS specialist at the Mayo Clinic in Rochester, Minnesota, said, “This has got to be one of the landmark papers. All that stuff that we were all taught has to be rethought.” Brian Popko, Ph.D., a neuroscientist and professor at the University of Chicago, agreed. “We thought two things—axons were either myelinated or not—and if they were, they were myelinated relatively homogeneously from initial segment out to the tip. This shows that there are axons that have large areas that are not myelinated, and … just knowing that now I think is critical.”
The researchers who were involved in the work were just as surprised and initially skeptical about their own results. “When I traced the first intermittent axons, I thought I had made some mistake, perhaps mistakenly jumped from one axon to another,” said first author Tomassy in an email to MSDF. “When [co-author] Daniel Berger [Ph.D.] and I independently confirmed all the tracings, then it became real; … a real shock! I immediately understood that was something bigger than I could ever [have] dreamed of!”
In an editorial accompanying the paper, R. Douglas Fields, Ph.D., of the U.S. National Institutes of Health’s National Institute of Child Health and Human Development wrote that it was time to “set aside the frayed metaphor” of myelin as a simple insulator and embrace its multifaceted roles in regulating and organizing the nervous system. “The findings are likely to spark new concepts about how information is transmitted and integrated in the brain,” wrote Fields, who was not involved in the work.
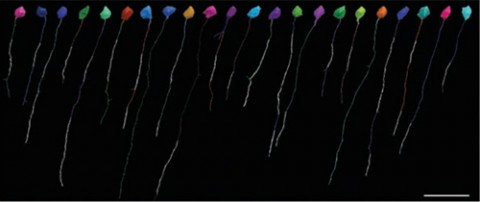
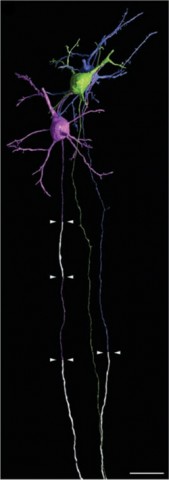 Those sparky findings came about thanks to three-dimensional reconstruction of electron microscopy sections of mouse pyramidal neurons of the cortex—nerve cells that are among the busiest in the brain—and their axons. After painstakingly reconstructing and coloring each neuron from serial sections, Tomassy and colleagues also mapped the precise distribution of myelin and found—in contrast to what everyone has thought—that different neurons have different patterns of myelination. The previous understanding was that if an axon was myelinated, the only gaps in otherwise complete coverage were the nodes of Ranvier. But Tomassy et al. found “intermittent myelin,” bits of myelinated axon interspersed among long segments of exposed axon many times the length of a node of Ranvier.
Those sparky findings came about thanks to three-dimensional reconstruction of electron microscopy sections of mouse pyramidal neurons of the cortex—nerve cells that are among the busiest in the brain—and their axons. After painstakingly reconstructing and coloring each neuron from serial sections, Tomassy and colleagues also mapped the precise distribution of myelin and found—in contrast to what everyone has thought—that different neurons have different patterns of myelination. The previous understanding was that if an axon was myelinated, the only gaps in otherwise complete coverage were the nodes of Ranvier. But Tomassy et al. found “intermittent myelin,” bits of myelinated axon interspersed among long segments of exposed axon many times the length of a node of Ranvier.
“What is intriguing is that neurons showing the most diversified profiles of myelination are the most ‘evolved’ neurons present in the brain,” said authors Tomassy and Paola Arlotta, Ph.D., in a joint comment emailed to MSDF. “Maybe as neuronal diversity increases and the brain needs to process more and more complex information, neurons change the way they use myelin.”
Tomassy and Arlotta added, “We were very fortunate to closely collaborate with the Jeff Lichtman lab (Harvard University), who developed the massive electron microscopy reconstructions for the cerebral cortex. We used a labeling software to literally navigate through those hundreds of sections and label, pretty much like in a color book for kids. The collaboration enabled this work and is an example of how collaborations across fields allow … great discoveries.”
According to Popko, the previous conceptualization about myelin distribution was that if an oligodendrocyte, the cell that myelinates axons, occurred near an axon, that axon was going to be myelinated. But “here the myelination is influencing the axon and vice versa in some kind of a feedback system, and maybe over time, as the nervous system matures, there will be changes,” he said. Tomassy and Arlotta point out that the myelin patterning was present even in older mice, suggesting that these myelin profiles are the end stage of the process.
Of course, a wide-open question relates to the function of this differential myelination. Jack Rosenbluth, M.D., a professor of neuroscience and physiology at New York University School of Medicine, suggested that unmyelinated regions of the axons could be involved in synaptic or nonsynaptic interactions with adjacent fibers, given that myelin would prevent such interactions. With the reduced conduction velocity wherever myelin is absent, Rosenbluth, who was not involved in the work, wrote in an email to MSDF, “the absence of myelin along portions of an axon could underlie changes in the synchrony or order of arrival of signals.”
Rodriguez agreed. “You’ve got myelin, you have no way of making contacts between an axon or a dendrite or another axon,” he said. “If you have a stretch that’s opened, maybe that’s the place where you have the interconnectivity.” That might allow for refined regulation and integration of inputs that total myelination wouldn’t, he said.
And, of course, when myelin is the subject, multiple sclerosis can’t be far behind. “The data suggest the intriguing possibility that different neurons may provide oligodendrocytes with different information about whether to myelinate or not,” wrote Arlotta and Tomassy. “It will be interesting to see how myelination changes during learning, or in the context of disease.”
Dwayne Godwin, Ph.D., a professor of neurobiology and anatomy at Wake Forest University School of Medicine, who was not involved in the work, wrote in an email that while the myelination patterns described in the study are a “developmental feature” rather than related to demyelination, “anything that tells us something new about the strategies used by the CNS [central nervous system] to control conduction velocity of neurons is incredibly important.”
To Rodriguez, the findings are more immediately relevant for MS, specifically in terms of how much clinicians rely on MRI to evaluate the disease in terms of inferred demyelination. “When we look at an MRI scan, we immediately say ‘Oh, that’s an area of demyelination,’ but that’s not true,” he said. He pointed out that when biopsied lesions from MS patients are examined, they show a lot of changes in macrophages and astrocytes, but often “the amount of demyelination is actually a very small part of the lesion.”
That links to the new findings, he said, because they show that even normal brains appear to lack myelination in many areas. “What this paper is saying is that these axons are not myelinated for long stretches. Billions of axons—you’d think the whole brain would be a checkerboard on MRI” if white spots on the MRI were really areas without myelination. “What we see by MRI scan cannot be demyelinating,” he argued. “What we see is water changes” because of inflammation.
An unexpected result like this one that might rewrite basic understanding opens many doors in many disciplines to multiple questions. “An obvious next step is to investigate how different myelination profiles affect function of neurons and circuits,” wrote Tomassy and Arlotta. “We like to think that our work provides basic information on how myelin is made and distributed during normal postnatal brain development to aid the identification of new molecules that mediate these events.” Pinpointing these molecules could lead to co-opting them to address demyelinating diseases, they said.
Popko didn’t want to speculate too broadly about future applications but noted what everyone seemed to agree on: “The most imp[ortant] thing to get out of this is that we didn’t know as much as we thought we did.”
Key open questions
- Will the findings be repeatable?
- Will other model species, including an outbred mouse strain or primates, show similar characteristic myelination patterns?
- How does myelination occur over time and what is the developmental trajectory?
- Who will rewrite the textbook?
Disclosures and sources of funding
Authors listed on this study were funded by the Howard Hughes Medical Institute, the Human Frontier Science Program, the Gatsby Foundation, National Institutes of Health grants NS062849 and NS078164, the New York Stem Cell Foundation, and the Harvard Stem Cell Institute. None of the commenters had anything to disclose.



Comments
Another key question : How does this fit with the idea of oligodendrocytes and trophic support of axons?