New NARCOMS Analysis Focuses on Symptom-Specific Impairment
A new symptom-specific analysis of data from the North American Research Committee on Multiple Sclerosis registry offers insights on MS progression
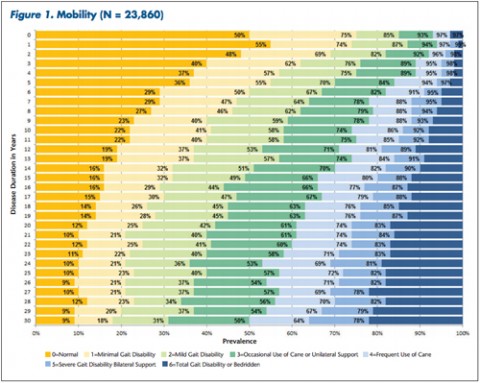
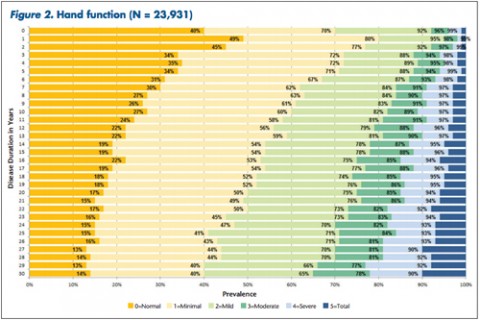
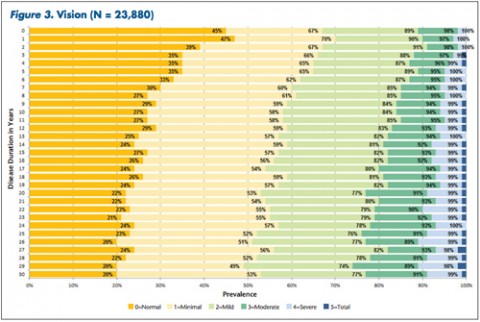
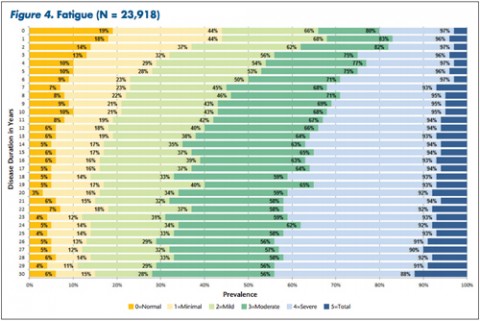
Patients and clinicians looking to assess comparative progression of MS-related disability in individual cases have a new tool. It not only charts general disability in a large cohort of MS patients over time, but also focuses on 11 specific domains of disability.
The new tool is based on data from more than 35,000 patients enrolled in the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry. An article detailing this new clinical tool appears in the fall 2013 issue of the International Journal of MS Care (Kister et al., 2013).
Ilya Kister, M.D., of the Department of Neurology, New York University, along with colleagues, used data from the NARCOMS Registry to compile reference tables that assess symptom prevalence and severity as a function of disease duration for each of 11 domains.
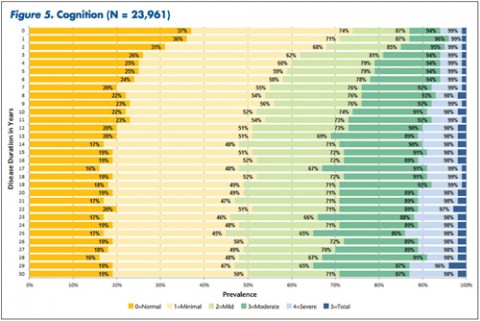
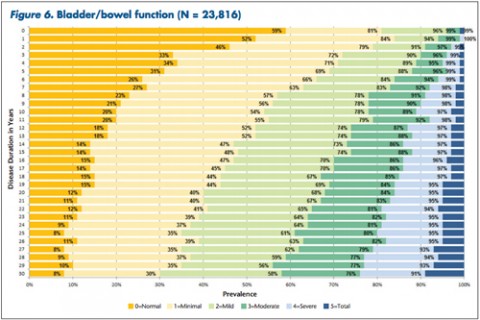
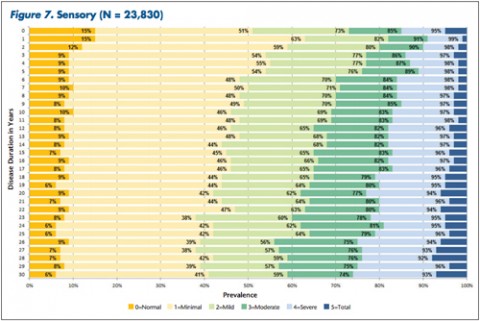
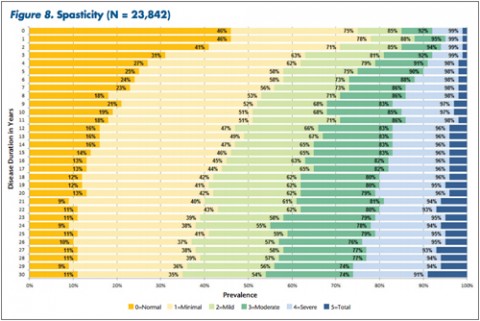
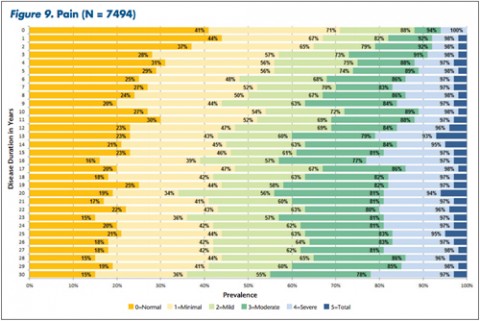
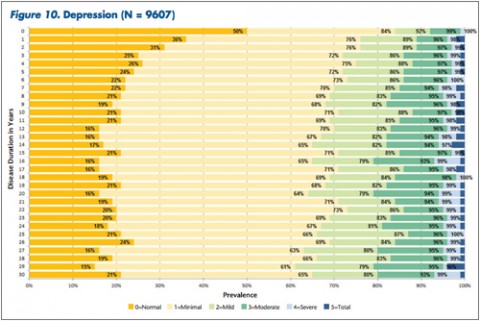
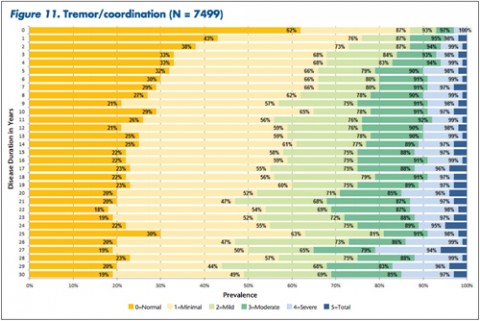
"The tables allow MS patients to easily determine how their impairment in mobility, hand function, vision, fatigue, cognition, bowel/bladder function, sensory, spasticity, pain, depression, and tremor/coordination domains compares to that of NARCOMS participants with the same disease duration," Dr. Kister and colleagues wrote.
The researchers included data from NARCOMS registrants who had been diagnosed with MS from 0-30 years previously. Most of the patients had been treated with various therapies. People with a disease duration of more than 30 years were excluded as their number was deemed too small for statistical analysis.
Based on their analysis, the researchers said that in nearly every domain, most patients reported some degree of impairment immediately after the onset of the disease. Sensory problems were particularly common early on, with 85% of patients reporting such issues. So was fatigue, reported by 81% of patients.
Cognitive impairment showed up during the first year in about half the patients.
The authors noted that mobility problems are generally thought to be relatively mild during the first decade of the disease. However, in this analysis, 35% of patients reported problems during the first year and an additional 15% said they sometimes needed to use a mobility device.
Overall, Dr. Kister and colleagues reported, "Worsening impairment was evident throughout the first decade for all 11 domains."
In some domains—namely, mobility, hand function, bowel/bladder function, and spasticity—increases in the proportion of moderate-to-severe impairment continued to mount steadily over the 3 decades of disease duration. In other domains, such as vision, cognition, sensory, pain, and depression, there was relatively little change in cumulative impairment during the third decade of disease, the authors said.
"Further study is warranted to ascertain whether the divergence in trajectories of disability accumulation in different domains is inherent to the nature of the disease or the result of measurement artifact," the authors wrote.
They emphasized that proper evaluation of MS patients, both in clinical practice as well as clinical trials, should include assessment across multiple domains.
They also said they think the symptom prevalence tables included in the article can improve understanding of MS among patients as well as clinicians. "We believe that the tables will provide optimal benefit if they are used in the context of clinician-patient discussion of an individual’s symptoms and their management," they conclude.
Key open questions
- This study included data on patients diagnosed with MS 0-30 years previously. How would data on patients diagnosed more than 30 years previously compare?
- The study, focusing on 11 different domains of disability, was based on data from the NARCOMS Registry, a volunteer cohort. How would data on the MS population at large compare?
Disclosures
This work was supported by a grant from the National Multiple Sclerosis Society (RG 4542-A-2) and by an unrestricted investigator-initiated research grant from Biogen Idec. The NARCOMS Registry is supported in part by the Consortium of Multiple Sclerosis Centers and its foundation. Dr. Kister has received research support from the National Multiple Sclerosis Society (NMSS). Dr. Chamot has received research support from the National Institutes of Health and NMSS. Dr. Cutter has received honoraria from GlaxoSmithKline, Biogen Idec, EMD Serono Inc., the National Institute of Neurological Disorders and Stroke, Consortium of Multiple Sclerosis Centers (CMSC), Novartis, Bayer Pharmaceuticals, and Teva Pharmaceuticals Industries Ltd.; has served on independent data and safety monitoring committees for Sanofi-Aventis, BioMS Medical Corp., GlaxoSmithKline, and Teva Pharmaceutical Industries Ltd.; has received research support from the CMSC (Director, NARCOMS Data Center) and NMSS; and serves as president of Pythagoras Inc. Dr. Herbert has received personal compensation for activities with Biogen Idec, EMD Serono, Teva Neuroscience, and Bayer as a consultant and has received research support from Teva Neuroscience, Novartis, Biogen Idec, Bayer, EMD Serono/Pfizer, BioMS, and INC Research. Ms. Bacon, Ms. Salter, and Ms. Kalina have nothing to disclose.


