More Than Meets the Eye
The promises and pitfalls of MRI imaging in multiple sclerosis
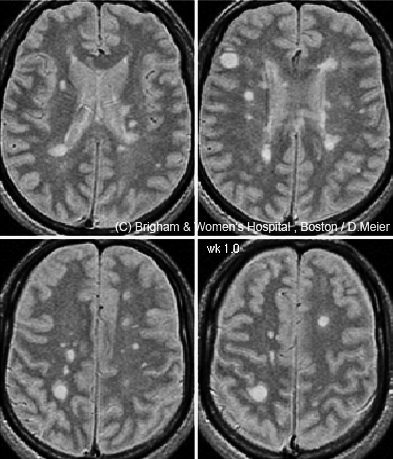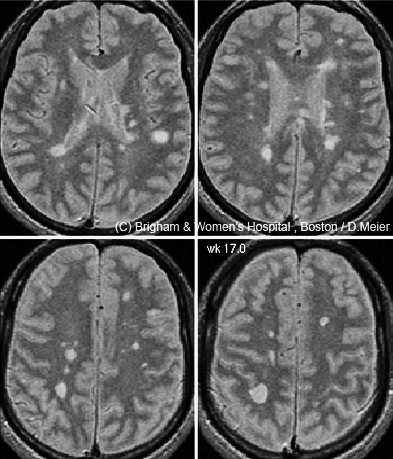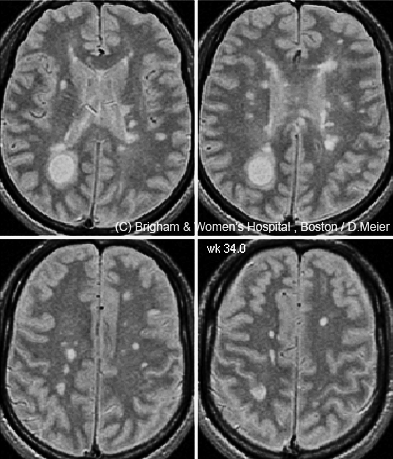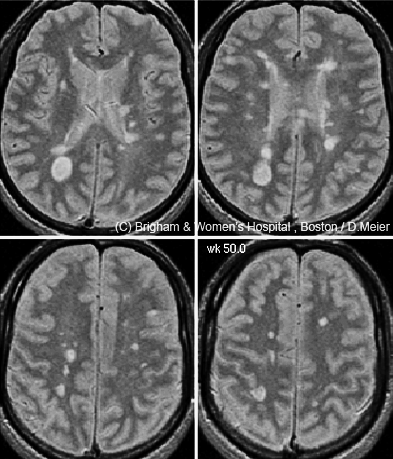
If a single magnetic resonance image is worth a thousand words, the time-lapse videos on Charles Guttmann’s laptop are worth a million. Each was created by a colleague, Dominik Meier, based on a time-series analysis of 24 brain magnetic resonance imaging scans taken in a man with relapsing-remitting multiple sclerosis (RRMS) over 12 months (Meier et al., 2007). Numerous abnormal bright spots blossom and grow or shrink, as if the disease is waging a fierce territorial war in the brain. “There is a great heterogeneity in size and course of these lesions,” says Guttmann, director of the Center for Neurological Imaging at Brigham and Women’s Hospital in Boston, where he works with Meier. And yet the 38-year-old man was, “according to the clinical measures, stable during that year,” Guttmann says.
MRI research over the past few decades has opened a visual window into the pathology and puzzle of MS, a scourge traditionally infamous for its attack on white matter of the central nervous system. Many investigators view MRI measures as the best prospect for a biological indicator—or biomarker—that can help them understand the disease process, diagnose patients, monitor treatment response, and predict prognosis. MRI tests have indeed sped up diagnosis, and they’ve become a ubiquitous tool in clinical drug trials.
Yet, shortcomings of current MRI technology have hindered it from fully living up to the imaging field’s high hopes, particularly as a prognostic tool. For starters, Meier’s time-lapse movies can’t tell anyone exactly what’s going on inside the patient’s lesions. Thus, to improve MRI’s value in MS, scientists are using more powerful and sensitive scanners, augmenting existing methods by combining them in new ways, and inventing next-generation techniques (Bakshi et al., 2008; Neema et al., 2007).
“Our ability to pick up the abnormalities has been limited by the technology in the past,” says Brigham and Women’s Hospital neurologist Rohit Bakshi. Now investigators are on the brink of various imaging-related advances that might allow them to peer more closely at the brain’s cells. With those innovations—plus cutting-edge imaging of the whole spinal cord and the recent epiphany that MS also ravages gray matter—the full scope of the damage is emerging.
A fundamental complication, however, is that MRI pictures and movies raise more questions than they answer. Intuition tells us that lesions, or plaques, can’t be good for the brain, but their meaning for a person’s functional status and future is unclear. The stumbling block is the so-called clinicoradiological paradox: Abnormal spots on MRI often don’t manifest in physical or cognitive symptoms. As the movies from Boston illustrate, brain scans can be red herrings, pinpointing neural-tissue changes that don’t match up with how a patient feels. And physicians cannot always trace symptoms to a particular spot on a scan.
Of all its uses in the MS field, MRI’s current ability to predict short- or long-term clinical outcomes is most controversial (Goodin, 2006). Some studies firmly lean one way or the other on the issue. “And there’s a whole spectrum of opinions in between,” says neurologist Robert Naismith of Washington University School of Medicine in St. Louis.
Sensitive in some ways, imprecise in others
MS-treating physicians and investigators rely upon conventional MRI scans, which include so-called T1-weighted or T2-weighted images, as well as gadolinium (Gd)-enhanced T1 images. Although brain lesions often don’t correlate with clinical symptoms, that discrepancy can partly be understood as a function of where in the nervous system those plaques hit, experts say, and of whether anatomical redundancies and adaptive mechanisms in a given person’s brain can compensate. But researchers add that MRI nonetheless offers a more objective measure of disease activity in MS—of what’s happening inside the brain—than clinical tests of, say, walking ability.
That’s because MRI is a highly sensitive tool: “It immediately detects if something changes in the brain, structurally,” Guttmann says. Conventional MRI identifies differences in water content. For instance, a white spot (or hyperintensity) on a T2 brain scan means excess water, which accompanies inflammation or swelling that can result from various causes, such as a tumor or infection—or an MS lesion. But the standard imaging techniques reveal only rough details of what underlies those changes, somewhat like a smoke detector that wails at the slightest whiff, but that doesn’t indicate whether the problem is a burning pot roast or an electrical fire.
MRI can’t parse what’s happening inside a lesion, Guttmann says. In MS, lesions arise when white blood cells, having crossed the blood-brain barrier, infiltrate an area and fuel inflammation that strips the fatty myelin insulation from the axon nerve fiber, thus letting additional water in to fill the breach, on top of the fluid of the initial swelling. Neurodegenerative processes gradually destroy the axons and nerve tissue to cause irreversible atrophy (and, later, to allow even more water to move in).
Thus, a bright, water-laden spot on a T2 scan in MS can represent different things, including inflammation, demyelination, and axon loss (Neema et al., 2007). MRI can’t even distinguish a neuron from other brain cells such as the oligodendrocytes that compose the myelin insulation. Complicating the picture further, the plaque is not necessarily all bad news: Within it, some remyelination can be occurring. T1 scans—which are like photo negatives of T2 images, with increased water showing up dark, not white—are likewise limited in the details they can provide. However, chronic black spots (T1 hypointensities, or “black holes”) indicate extreme and permanent tissue destruction of both myelin and axons.
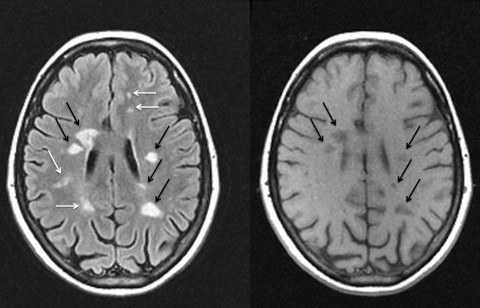
Injecting gadolinium, a contrast dye, into veins before taking T1 scans allows detection of fresh, so-called Gd-enhanced lesions. (The method can also be applied with T2 scans.) Because the blood-brain barrier opens only temporarily in the early days to weeks of a lesion’s formation in MS, the dye can flow into, accumulate in, and highlight new plaques, but not old ones.
Given the constraints of conventional MRI, researchers have been experimenting with next-generation “quantitative” MRI methods that promise to show details of the disease process within brain cells, such as the amount of myelin loss or axon destruction—or even subsequent repair—inside lesions. These techniques include diffusion tensor imaging (DTI), magnetization transfer imaging, and magnetic resonance spectroscopy, among others. (See “Can next-generation methods help fill in the gaps?”)
Diagnosing MS faster
Despite their limitations, conventional MRI techniques have made it easier for clinicians to finger MS since 2001. That year, imaging measures were first incorporated into the McDonald diagnostic criteria, which have since become the gold standard worldwide for identifying the illness.
In decades past, if a patient experienced an initial neurologic attack (called a clinically isolated syndrome), doctors had to wait for a second episode before they could confirm MS. With the 2001 criteria, however, that diagnosis could be clinched if brain images taken a few months apart revealed that new lesions had formed. “That allows patients to be given a definitive diagnosis of MS much earlier, so they can start therapy,” says neurologist Nancy Sicotte of Cedars-Sinai Medical Center in Los Angeles, California. And under criteria revised in 2010 (Polman et al., 2011), a single Gd-enhanced scan can confirm MS in some cases. For diagnosis, clinicians are relying more and more on imaging—and most experts agree that it has benefited patients.
MRI for monitoring and treating MS: A work in progress
But beyond diagnosis, MRI’s utility is less certain: Its helpfulness has not been clearly established for tracking disease course and treatment responses. For one thing, in routine clinical practice, doctors typically monitor MS patients with T2 and Gd-enhanced scans taken every year or two (or every 6 months in very active cases)—but researchers know that these scans, using standard MRI analysis methods, can miss a lot of disease activity (Cotton et al., 2003; Meier et al., 2007). That lesson comes from MS research centers, such as Guttmann’s, where patients often undergo imaging more than once a year and where advanced image-analysis software tools have made it easier to align and compare a person’s brain scans taken at different visits.
A more sensitive method can now “subtract” an older scan from a newer one to identify recent changes in the brain, Guttmann says, provided that the images were taken with similar scanners and protocols. Any difference identified by this technique, called subtraction imaging (sMRI), immediately “bangs you in the eye,” he says. In a small study, Guttmann and colleagues in Boston and Amsterdam found that two-thirds of MS patients showed no Gd-enhanced lesions at their initial visit or 1 year later (Liguori et al., 2011). If clinicians relied on Gd-enhanced scans as a primary indicator of active disease, they might conclude that those patients were stable, Guttmann says; although doctors also look for whether new lesions have formed on T2 scans since the previous year’s visit, he adds, they tend to eyeball the images in a cursory manner. sMRI makes the comparison more precise by automating it. In Guttmann’s study, 83% of those apparently “stable” patients showed disease activity on subtraction imaging of T2-weighted scans taken at the same two time points.
Clinicians traditionally view plaques detected on T2 images—or on another type of conventional T2-weighted scan called fluid-attenuated inversion recovery imaging (FLAIR)—as a fixed record of disease. But many of those lesions can heal in such a way that they disappear from that record, according to unpublished results from Naismith and Stuart Cook of the University of Medicine and Dentistry of New Jersey in Newark (Qian et al., 2011). Tracking 75 RRMS patients, the team took monthly MRIs of various types—such as T1, T2, FLAIR, and DTI—on an advanced brain scanner with a magnet twice as powerful as in conventional machines (3 tesla versus 1.5 tesla). The stronger magnetic field provided a greater ability to identify differences in water content and contributed to the researchers’ highly sensitive technique for optimizing detection of new brain inflammation: They looked for enhanced T1 lesions after giving a triple dose of Gd and letting it circulate for 40 minutes instead of the usual 5- to 10-minute wait.
Over the course of 2 years, the investigators observed hundreds of new lesions form in the patient group. But more than 30% of the FLAIR lesions disappeared within several months, Naismith says. However, an examination of tissue integrity within those lesions—using DTI—revealed that traces of injury persisted (although leaving less damage than in permanent FLAIR lesions). Lesions that were transient on FLAIR persisted longer on other types of T2 scans, but a significant subset of plaques still vanished from detection by those techniques too.
Such findings are disturbing, says neurologist Benjamin Greenberg of the University of Texas Southwestern Medical Center in Dallas (who is an Accelerated Cure Project scientific adviser). Many of his own MS patients on treatments look good, feel good, and have T2 lesions that appear stable on MRI from year to year, but they could have plaques “coming and going, coming and going, and I just didn't catch 'em,” he says. Do patients with such fluctuating activity fare worse over time than others? “We are definitely looking into that,” Naismith says. “While it is good that lesions disappear and are associated with less tissue injury, I don’t think we can be sure that the region has returned to ‘normal.’ ”
Although no medical practice guidelines exist for how to optimally adjust a patient's therapy based on MRI changes, clinicians at research centers including the National Institute of Neurological Disorders and Stroke (NINDS) in Bethesda, Maryland, rely heavily on frequent imaging to assess whether treatments are working. Clinical trials have shown that when drugs reduce relapses of MS symptoms, lesion activity on scans decreases, says neurologist Daniel Reich, chief of the translational neuroradiology unit at NINDS. So if imaging of a patient shows many more plaques appearing, Reich says, that individual probably is not benefiting from a medication and should consider switching to another drug.
Predicting MS outcomes: A tough quest marked by dissension
Many people cope relatively well with typical early-stage RRMS: They often recover fully from relapses and can take drugs that reduce the frequency of these attacks. What patients tend to fear most is the potential for permanent disability that confines them to a wheelchair, Reich says. But disability from MS is unpredictable and takes years or decades to emerge.
Thus, the ultimate vision is that MRI scans could someday help forecast whether an individual newly diagnosed with MS will experience a significant physical decline in, say, 5 or 15 years or sail through the next 3 decades without trouble, and whether treatment might change that prognosis. But right now, controversy persists about how helpful conventional MRI scans are in projecting disability. “When you look at the data in terms of what MRI is able to predict, it’s humbling,” Naismith says.
Starting off with group-level, short-term predictions
The difficulty is finding a strategy sensitive enough to read the crystal ball for a given person with MS. Research so far has mostly focused not on individual patients but on groups of them, which is plenty challenging in itself. Evidence indicates that on average, people with MS who suffer many clinical attacks or show a large burden of lesions on MRI early in their disease tend to fare worse, years later, than those who don’t. In addition, many clinical studies and drug trials in MS have found simple correlations, when analyzing averaged results from groups of patients, between conventional MRI measures and relapse rates or scores of disability on the Expanded Disability Status Scale (EDSS). However, the EDSS itself is generally considered an insensitive short-term yardstick (Ebers et al., 2008). And because of expense and participant dropout, most MS studies follow patients for only 2 or 3 years—not long enough for substantive disability to emerge.
Moreover, “simple correlations [between MRI measures and clinical outcomes] are not what you want,” says George Ebers, a neurologist who studies MS epidemiology and genetics at Oxford University in the U.K. To justify the time and expense of costly MRI scans, he says, the important question is whether imaging contributes value “independently of everything else” in predicting disability, particularly in the long run. The answer is “no” for changes in T2 lesion volume and Gd-enhanced lesions, say Ebers and Martin Daumer, director of the nonprofit Sylvia Lawry Centre for Multiple Sclerosis Research in Munich. They drew this conclusion from a multivariate analysis of data on untreated patients from 31 clinical trials that documented those conventional MRI markers (Daumer et al., 2009; Ebers 2010; Koch-Henriksen, 2009). The strongest predictors of short-term, on-study relapse rate or EDSS changes were patients’ disease duration and past relapse rate.
“In a nutshell, MRI as it is used is not a good investment” for predicting outcomes, Daumer says. (Nor does it have value for monitoring patients, he says, as it adds little information beyond symptoms or relapses.) Advanced methods might be better, he says, but must be proven.
Other scientists dispute the Sylvia Lawry analysis because of heterogeneity in how MRIs were acquired and analyzed in the assessed studies (Sormani, Filippi et al., 2009). Pooling data sets “is very dangerous if you cannot control for all the sources of variability,” says statistician Maria Pia Sormani of the University of Genoa in Italy. (Worth noting, though, is that inconsistencies in MRI data collection have also been an issue even for single multicenter clinical trials; see “Getting coordinated in the MS field.”) Sormani’s own meta-analyses of pooled data from MS trials—which she says were properly controlled and included treated as well as untreated patient groups—support a significant correlation between a therapy’s effect on MRI measures and its effect on relapses and EDSS worsening during the short time frame of the studies, at a group level (Sormani, Bonzano et al., 2009; Sormani et al., 2010). Last year, she and colleagues extended those findings to the individual-patient level (Sormani, Stubinski et al., 2011; Sormani, Li et al., 2011). Many of their studies have demonstrated simple correlations, however, and do not satisfy Ebers’s desire to see a demonstration that MRI provides predictive value independent of other indicators.
The arguments and counterarguments about predicting outcomes with MRI can be downright dizzying. But even if further short-term studies definitively confirm that brain-scan changes can reliably forecast that a particular patient on drug therapy will, in the next year or two, suffer new relapses or EDSS worsening, they still wouldn’t be able to prove that MRI can project that person’s odds of permanent disability 10 or 20 years later.
Trying to gaze far into the future
Long-term MS studies are expensive and difficult; they are thus scarce and tricky to interpret. But in December 2011, neurologist Douglas Goodin of the University of California, San Francisco, published a 16-year follow-up analysis of 260 MS patients in the clinical trial of interferon-β-1b that led to the drug’s 1993 approval by the U.S. Food and Drug Administration (FDA). Working with Ebers and others, Goodin determined in a multivariate analysis (at a group level) that T2-lesion and brain atrophy changes during the original 3-year trial did not independently correlate with the accumulation of severe physical disability at 16 years (Goodin et al., 2011).
The 16-year report is likely to stir more debate—particularly because on-study T2-lesion measures have already gained widespread use in the process of testing and approving MS drugs in the United States (see “A shortcut to speed drug development”). Yet, in Goodin’s view, the analysis shows that such measures are “pretty weakly associated” with long-term physical function, he says. (In contrast, baseline MRI status at the start of the interferon trial correlated better than the MRI changes documented during the following 3 years—but still not strongly enough to accurately predict physical disability, he notes.)
Others say the researchers’ conclusions are premature. After the initial interferon trial, many things could have happened in the patients’ treatment histories that might muddy the picture, Sormani says. For example, those who originally received a placebo treatment were later offered interferon; any differences in MRI lesions that the drug triggered between the treated and untreated groups during the trial’s 3 years would tend to disappear, since everyone ended up on interferon. After 16 years, all correlations “tend to be diluted,” Sormani says, making it harder to detect any predictive power of MRI for long-term events. “It’s much easier to lose a correlation rather than to demonstrate it.”
Goodin acknowledges that his analysis represents only one data set, includes only two time points, and needs to be replicated. Yet, the broader point, he says, is that the MS imaging community has “a technique that they’ve really never properly validated” as a proxy for long-term disability—the ultimate outcome that MS patients, doctors, and investigators care about.
On the other side of the spectrum, neurologist Jerry Wolinsky of the University of Texas Health Science Center at Houston says “MRI is probably the most important biomarker we have for evaluating multiple sclerosis, period.” Clinical measures such as MS disease duration—which stood out as good predictors in Daumer and Ebers’s 2009 meta-analysis—are “pretty fuzzy when you get down to what they’re based on,” Wolinsky adds, as many individuals aren’t diagnosed until years after what they realize, in retrospect, was probably their first attack.
Still, the need for robust predictors of longer-term outcomes remains a major problem for the field, Wolinsky agrees: “I don’t know how we get around it until we start running the 5- and 6-year studies.” Meanwhile, the imaging field’s search for a prognostic biomarker has shifted toward measures of brain atrophy—and atrophy in gray matter, in particular—which might predict disability better than inflammatory lesion activity (Fisher et al., 2000; Fisniku et al., 2008).
A shortcut to speed drug development
Although the value of Gd-enhanced and T2 lesions as clinical biomarkers for future disability is still disputed, trials of drugs for MS typically track both of these MRI measures in the short term, particularly as a gauge of relapse rate. As many studies, including Sormani’s work, have shown, inflammation-inhibiting medications such as interferon that suppress new lesions on conventional MRI generally curb the number of relapses in the 2- to 3-year time frame of clinical trials. That being the case, Sicotte says, an experimental anti-inflammatory agent that fails to reduce new Gd-enhanced lesions in small, early-stage studies might not be developed further. This practice saves the effort and cost of proceeding with a compound that probably will not benefit patients. In contrast, positive early results give the green light for the larger, longer phase III trials for establishing a drug’s clinical benefits to win FDA approval.
Nearly all MS drug trials now also track changes in whole-brain tissue volume as a gauge of atrophy. Brain volume gradually dwindles in MS, but measurements can be confounded by other processes, says neurologist Richard Rudick of the Cleveland Clinic in Ohio. For example, inflammatory activity of the disease can swell the white matter and increase its volume, whereas beneficial drug therapy can shrink it by quelling inflammation. A crucial unanswered question is how MS treatments affect abnormalities in gray matter, which are more extensive in MS than experts once thought (see “Revealing what’s below the tip of the MS-pathology iceberg”). Few clinical trials specifically monitor gray-matter pathology because researchers are still struggling to image it, Rudick says.
In the next couple of years, Rudick and others say, the FDA might move toward accepting the use of T2 lesions and Gd-enhanced lesions as primary surrogate markers of relapse-reducing efficacy when assessing drugs—starting with generic forms of anti-inflammatory therapies. If a generic cousin of interferon shows similar effects on MRI to the already-approved drug, “why would you need to do a long, expensive study to show that it has clinical benefits?” Rudick says. However, regulatory agencies are unlikely to accept those MRI measures as universal surrogate markers when it comes to testing new medication classes that work through different mechanisms, Wolinsky says. It’s possible that some novel therapies might not inhibit MRI lesion activity, yet could still quell flare-ups by, say, counteracting the downstream consequences of the plaques; or they could protect neurons in a way that prevents disease progression. In such scenarios, investigators and drug developers would need to come up with more relevant predictors of clinical benefit in phase II testing—or take a leap of faith in proceeding to phase III studies. Meanwhile, some researchers, such as Ebers and Daumer, who question MRI’s ability to predict disease course, still regard conventional imaging measures as far from validated surrogate markers of any clinical outcomes in drug trials.
Revealing what’s below the tip of the MS-pathology iceberg
While that debate simmers, advanced imaging tools are allowing researchers to glean deeper insights into the biology of MS (Bakshi et al., 2008; Geurts, 2008; Neema et al., 2007). Quantitative MRI studies have identified diffuse microscopic damage in what looks like normal brain tissue on conventional MRIs; experts are still investigating its clinical significance.
Common wisdom holds that disease activity in the brain reflects disease activity in the spinal cord, so it isn’t necessary to scan the spinal cord often. But as scientists have devised better ways to image and rapidly analyze the whole spinal cord, says Brigham and Women’s Bakshi, they are seeing that lesion volumes and amount of atrophy in the spinal cord do not correlate with disease activity in the brain (Horsfield et al., 2010; Cohen et al., 2011). The findings suggest that structural damage unfolds independently in the two areas—meaning that even when no new changes show up on brain scans, MS could be progressing in the spinal cord without the patient knowing it, Bakshi says. “You really have to comprehensively image these patients to find out how they're doing,” he says. Spinal cord disease in MS might partly account for the mismatch between brain MRI measures and current (or future) clinical status.
Likewise, another potential explanation for the clinicoradiological paradox is that white-matter lesions in the brain are just the tip of the iceberg in MS. Although experts traditionally thought that the illness primarily targets white matter, which is mostly made up of myelin-covered nerve axons, the brain’s gray matter contains not just nerve cell bodies but plenty of axons too. The recognition that MS also triggers substantial demyelination and neuronal injury in gray matter—in the cortex and certain deeper structures—has made the research area a hotbed of inquiry (Hulst and Geurts, 2011). Gray-matter pathology is “the Trojan horse of multiple sclerosis,” Rudick says.
Unlike white-matter lesions, gray-matter lesions often aren’t visible on conventional T2 scans because they don’t involve as much infiltration by inflammation-provoking white blood cells, Sicotte says. It was only by using FLAIR scans (which adjust T2 scans for the brightness of background water signals from fluid in nearby brain ventricles) that investigators first noticed cortical lesions. Improved histological staining techniques confirmed that the cortex is “chock-full of demyelinated areas,” Sicotte says. A newer MRI method called double inversion recovery (DIR) provides a more sensitive way to find gray-matter lesions.
Cortical lesions may be “extremely important” as an underlying cause of disability and disease progression, Rudick says. In addition, measures of gray-matter atrophy seem to offer the best MRI correlate of long-term progressive disability in MS, compared with T2-lesion load or white-matter atrophy, according to research that includes studies published in 2008 from the Cleveland Clinic and University College London (Fisher et al., 2008; Fisniku et al., 2008; Geurts, 2008). The field is now heading “as fast as it can go” toward developing sensitive measures of gray-matter pathology, Rudick says. (Although DIR is better at catching cortical lesions than other methods, for instance, it still leaves room for improvement, he says.) “This is probably one of the most urgent needs in the MS field.”
By combining old and new methods, many imaging researchers are now working to develop multimodal, global neurological assessments of the MS patient in hopes of boosting MRI’s prognostic power. For example, Bakshi, colleague Mohit Neema, and other associates are testing a tool called the Magnetic Resonance Disease Severity Scale (MRDSS) on a 3-tesla scanner platform in 200 patients to determine whether it can predict EDSS status, cognitive function, and quality-of-life changes 5 years later (Moodie et al., 2012). The latest version of MRDSS will factor in white-matter abnormalities by measuring T2 lesion and T1 black hole volumes as well as diffuse damage (as detected by diffusion tensor imaging). But the tool will also scrutinize atrophy, from T1 scan data, in the brain’s gray matter as well as in the spinal cord.
Can next-generation methods help fill in the gaps?
Everybody agrees that conventional MRI tells just part of the story of how MS patients are faring. It’s like watching a movie about a complex crime from the viewpoint of the most obvious suspect. The complete story, encompassing an entire gangster ring, would entail not just whether and where T2 or Gd-enhanced lesions are present, but also whether other kinds of damage are ravaging the brain or spinal cord. “If we could see all those abnormalities and we could control them”—by developing effective drugs—“then I think we would be doing a great service for patients,” says Reich at NINDS.
To that end, investigators are evaluating quantitative MRI measures that—unlike conventional brain scans, which focus on lesions and atrophy—can derive more detailed information about myelin damage and subsequent repair (Bakshi et al., 2008; Neema et al., 2007). For instance, DTI can track the movement of water within brain tissue. Water molecules generally diffuse within the lengths of healthy axons but tend to move outward (perpendicular to the axon fibers) if nerve tissue has been damaged. Scientists thus are trying to harness DTI as a way to assess myelin or axon integrity in the brain and spinal cord. Reich has been using DTI to explore the links between disability and tissue damage in specific nerve tracts. However, DTI is still experimental and limited by the fact that “we don’t quite know what we’re measuring on the tissue level,” he says.
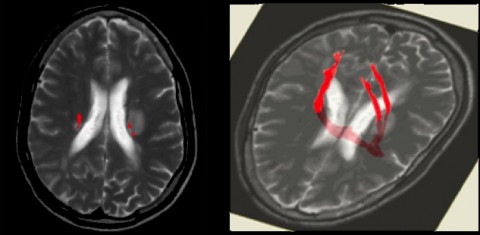
By comparison, magnetization transfer MRI gauges myelin integrity in brain tissue by measuring how much water is linked with myelin (which, despite its high content of fatty molecules, contains some water) rather than unattached. Demyelinated areas show increased amounts of “free” water; the less water-linked, healthy myelin present, the lower the magnetization transfer ratio (MTR). Some experts say that this technology could be a good way of quantifying remyelination; others aren’t yet convinced.
Improvements to MTR are in the works, however, and a novel method might yield more accurate measurements, says neurologist Robert Zivadinov of the University at Buffalo School of Medicine and Biomedical Sciences in New York. The approach scrutinizes myelin increases or decreases in each voxel, or three-dimensional pixel, within an MTR brain map, rather than taking an average of the changes in a region of interest as other, histogram-based analysis methods do. As such, Zivadinov says, the voxel-wise MTR strategy is more sensitive to detecting demyelination and remyelination within an area—competing processes that could cancel each other out if regionally averaged on MTR images (Dwyer et al., 2009). By tracking myelin changes, MTR reveals “another aspect of the disease that might be extremely important, especially as we move from drugs that are stopping the inflammation to drugs that are promoting remyelination,” he says.
Meanwhile, magnetic resonance spectroscopy uses standard MRI scanners to detect other molecules, such as N-acetyl aspartate (NAA), choline, or creatine. The approach reveals useful information that conventional MRI cannot. Declining NAA levels, for instance, reflect neuronal death. But one downside of spectroscopy and MTR, Sicotte says, is that they require a fair amount of postprocessing data analysis, which radiologists usually don’t have time for in the clinic.
Other types of imaging technology are also emerging. For instance, optical coherence tomography can spot changes in optic nerve fibers, which are normally unmyelinated and thereby offer the potential for studying axonal neurodegenerative processes rather than demyelination (Galetta et al., 2011; Petzold et al., 2010). Even MS patients without visual symptoms can have progressive optic nerve thinning, research suggests; if so, the technique could provide a general read on axonal health.
Getting coordinated in the MS field
Advanced imaging techniques have been evolving so quickly that the field is “moving faster than our ability to validate the findings,” Greenberg says. Correlations with tissue pathology and long-term studies are needed to confirm the meaning and prognostic significance of these measures—which take considerable time and money.
Instead of a “free-for-all” where everybody is racing to pursue the hottest new methods without pausing to do a thorough assessment of their utility, MS imaging leaders should identify the most promising MRI techniques and then validate them, Greenberg says. He suggests they work with the FDA, the National Institutes of Health, and the U.S. National Multiple Sclerosis Society to get uniform imaging protocols into clinical trials, so that MRI results can be gathered and pooled across different studies for analysis.
Indeed, inconsistencies in MRI data gathering have challenged the imaging field because scan acquisition isn’t identical from site to site, even within a multicenter clinical study that uses a single MRI analysis center. But solving the dilemma is easier said than done. To fully exploit MRI’s potential as a screening and prognostic tool would require a major effort to standardize equipment, acquisition protocols, and image-analysis software across manufacturing vendors, says Daumer of the Sylvia Lawry Centre. In the past, the three leading MRI equipment makers expressed interest in such a project, which would cost roughly $50 million, but the center lacks funding to do it, he says.
Although they fall short of that kind of comprehensive endeavor, the imaging community has undertaken other efforts toward setting some MRI standards in research: In January 2012, an international panel published recommendations based on a workshop held by Magnetic Imaging in Multiple Sclerosis (MAGNIMS) of Europe, the U.S. National Multiple Sclerosis Society, and the Multiple Sclerosis Society of Canada, with funding from Bayer Schering Pharma (Barkhof et al., 2012). In the United States, the Common Data Elements (CDE) Project—sponsored by NINDS—aims to establish standards for collecting data in clinical studies of neurologic diseases. But standardizing an imaging approach might “fossilize” it before it has fully evolved—which could mean losing an opportunity to learn new things, says Wolinsky, chair of the imaging subgroup for the MS CDEs. Thus the committee must strike a balance. Whether the balance is the right one is an issue of debate. The panel released its proposed imaging CDEs to public comment in January and February 2012; version 1.0 of the standards will be published in April 2012.
Meanwhile, a largely unaddressed concern in the field—raised by Ebers and Daumer—is that widespread partnerships and financial ties between academic investigators and the pharmaceutical and MRI imaging industries have created a bias in research findings that favors MRI’s usage, particularly in drug testing (Ebers, 2010). Such potential conflicts of interest have become common across the clinical research world, which continues to grapple with them. (See the end of this article for disclosures of industry ties for the experts interviewed for this story.)
Visualizing the future of MS imaging
In the next 5 to 10 years, MS community clinics will shift from 1.5-tesla to 3-tesla scanners, which will mean faster scan times and a more sensitive and accurate depiction of the disease process, Bakshi says. Some research labs, meanwhile, are ugrading to 7-tesla machines. A second trend will be a move toward assessing gray-matter damage in routine clinical imaging. The development of sensitive gray-matter MRI measures, Rudick says, “will have major implications in understanding the disease, diagnosing it, making prognoses, and developing drugs for progressive MS.” It should also become clearer which of the advanced imaging methods will pay off, even as various other, still-fledgling imaging techniques mature in research labs, such as myelin water fraction—another way to measure myelin—and perfusion MRI, which aims to identify brain blood-flow changes associated with lesions or disease evolution.
Whichever imaging tests prove valuable in the clinic, MRI’s contribution must be viewed as part of the broader picture. “It’s a complex disease,” says Guttmann in Boston. “MRI continues to be a useful tool, but it’s not a panacea” for illuminating the mysteries of MS. To fathom how the affliction manifests itself in patients, he says, researchers must consider imaging findings along with genetic, environmental, and other factors. MRI technology has transformed the practice of medicine for MS in accelerating diagnoses and helping to inform some treatment decisions, but it “doesn’t show us everything, and sometimes it shows us things that are not important,” says UT Southwestern’s Greenberg. “We have to put it into context.”
Key open questions
- Conventional MRI scans highlight white-matter lesions that often do not correlate with a patient’s physical symptoms, a mismatch known as the clinicoradiological paradox. Recent advances in MRI techniques have allowed better detection of damage in gray matter and the spinal cord. Do abnormalities in those areas correlate better with clinical status?
- In a paradigm shift, research has shown that MS is not merely a white-matter disease; gray-matter damage in the cortex is also often extensive. What are the best imaging techniques for spotting and quantifying gray-matter lesions and atrophy and tracking how much those change over time?
- How do current MS drug therapies affect gray-matter pathology, and can MRI methods accurately detect those effects?
- Can combinations of conventional and/or advanced MRI metrics be validated as reliable, independent, and cost-effective measures for predicting an MS patient's long-term prognosis for developing disability?
- Should regulatory agencies accept MRI measures as official surrogate markers in clinical drug trials, permitting their use as primary endpoints that demonstrate a clinical benefit in reducing relapses or disability progression in MS?
- What imaging tools might be developed to quickly identify patients who are not responding to a drug therapy, so that they can be switched to another medication?
- Researchers are working on next-generation MRI techniques that promise to quantify specific types of tissue pathology within MS brain lesions. Can these quantitative methods provide reliable measures of the amounts of myelin loss or nerve damage?
- Differences in how MRI scans are acquired and analyzed create challenges in pooling and assessing data from different clinical studies and drug trials. What standards should be set for MRI protocols and image-analysis software in research to allow for meaningful comparisons among data sets?
- MS is a complex disease that appears to be influenced by numerous genetic and environmental factors, including key immune-system genes and vitamin D levels. How do these factors correlate with MRI measures of MS activity, and what might that reveal about mechanisms of the disease?
Image credits
Thumbnail image on landing page. Courtesy of Daniel Reich, NINDS.
Movie. Courtesy of Dominik Meier, Brigham and Women's Hospital. For details on the time-series analysis method behind these movies, see Meier et al., 2007.
Fig. 1. Courtesy of Daniel Reich, NINDS.
Fig. 2. Courtesy of Daniel Reich, NINDS.
Conflict-of-Interest Disclosures as of February 2012
The following is a list, not necessarily complete, of recent industry-related disclosures for experts interviewed for this MS Discovery Forum News Synthesis article, as reported in their research publications. MS Discovery Forum is funded with a grant from EMD Serono, an affiliate of Merck KGaA, to Massachusetts General Hospital. The forum operates completely independently of its funders, who have no influence over the site's content or operations.
Rohit Bakshi of Brigham and Women's Hospital has received consulting fees from Biogen Idec, Novartis, Questcor, and Teva Neuroscience. He has received research support from Biogen, EMD Serono, and Teva Neuroscience.
Martin Daumer is scientific director of the Sylvia Lawry Centre and a managing director of Trium Analysis Online GmbH. The Sylvia Lawry Centre is a medical adviser to the German Multiple Sclerosis Society and has received honoraria for consulting, statistical analysis, and use of actibelt® technology from entities including Bayer Schering Pharma, Biopartners, Biogen Idec, BioGeneriX, Böhringer Ingelheim, Eisai Limited, HERON Evidence Development, Hoffmann-La Roche, Johnson & Johnson Pharmaceutical Research & Development, Sanofi-Aventis U.S., and Novartis Pharma GmbH.
George Ebers of Oxford University has participated in advisory boards for Novartis and Bayer HealthCare, received speaking fees from Sanofi-Aventis, and consulted for Bayer, Howrey LLP, HERON Health, Eli Lilly & Co., and UCB Pharma. He has received research grant support from Bayer Schering Pharma.
Douglas Goodin has received fees from Merck Serono, Novartis, Berlex Laboratories, Bayer Pharmaceuticals Corporation, Biogen Idec, Schering AG, and Teva Neuroscience for speaking, consulting, and work on clinical trials. He has received research support from Bayer HealthCare and Novartis.
Benjamin Greenberg of the University of Texas Southwestern Medical Center has received honoraria or consulting fees from Biogen Idec, EMD Serono, Sanofi-Aventis Pharmaceuticals, and Teva Neuroscience. He has also consulted for DioGenix and holds equity in that company. He has received research support from Amplimmune.
Charles Guttmann of Brigham and Women’s Hospital has received research support from Teva Pharmaceutical Industries, has been a scientific board adviser to Johnson & Johnson, and has consulted for and received speaking fees from Biogen Idec, Merck Serono, Teva Neuroscience, and Pepgen. He holds ownership interest in Roche and Novartis.
Robert Naismith of Washington University in St. Louis has received speaking or consulting fees and/or travel expenses from Acorda Therapeutics, Bayer Schering Pharma, Biogen Idec, Elan Corporation, EMD Serono, Genzyme Corporation, Questcor, and Teva Pharmaceutical Industries. Naismith has received research support from Acorda Therapeutics, Biogen, and Genentech.
Daniel Reich of the National Institute of Neurological Disorders and Stroke has no industry-related conflicts of interest to disclose.
Richard Rudick of Cleveland Clinic has served as a consultant for Bayhill Therapeutics, Biogen Idec, Genzyme Corporation, Millennium Pharmaceuticals, Pfizer, Teva Neuroscience, and Wyeth Pharmaceuticals. He has also received research support from Biogen Idec and Elan Corporation.
Nancy Sicotte of Cedars-Sinai Medical Center in Los Angeles, California, reported having no industry-related conflicts of interest to the American Academy of Neurology annual meeting disclosures database.
Maria Pia Sormani of the University of Genoa has served on a scientific advisory board for Biogen Idec and has received consulting or speaking fees from Actelion Pharmaceuticals, Biogen, Merck Serono, Synthon, and Teva Pharmaceutical Industries.
Jerry Wolinsky of the University of Texas Health Science Center at Houston (UTHSCH) has been a member of advisory boards or data monitoring committees for Eli Lilly, Novartis, Sanofi-Aventis, Teva Pharmaceuticals, and UCB. He has consulted for or received speaker fees from Acorda Therapeutics, Actelion Pharmaceuticals, Bayer HealthCare, Biogen Idec, EMD Serono, Facet Biotech, Hoffmann-LaRoche, Novartis, Peptimmune, Pfizer, Sanofi-Aventis, Serono Symposia International Foundation, Teva Neuroscience, and Teva Pharmaceuticals. Wolinsky has received research or contractual support from Sanofi-Aventis and the NIH as principal investigator of a subcontract to UTHSCH for image analysis in MS drug trials. He also receives royalties from Millipore (Chemicon) for monoclonal antibody technology.
Robert Zivadinov of the University at Buffalo School of Medicine and Biomedical Sciences has consulted for or received speaker honoraria from Biogen Idec, EMD Serono, Genzyme, Questcor, and Teva Neuroscience. He has received research support from Biogen, Teva, Genzyme, Questcor Pharmaceuticals, Bracco, EMD Serono, and Greatbatch.


