Image-Ready
Meeting notes: Imaging neurodegeneration and repair in MS
NEW YORK CITY—It’s 9:30 on a Friday morning and, from the podium, a man is telling me and 150 or so other people to stand up, put our hands in the air, spin around, and pretend we’re protons. Acting class warm-up? Nope, a conference on imaging neurodegeneration and repair in MS, held at the New York Academy of Sciences on June 15 and 16. The meeting covered a lot of ground, from new imaging methods that offer improved myelin specificity to the usefulness of imaging different areas of the central nervous system. The 1.5-day gathering had a collegial feel as attendees grappled with issues such as what white and gray matter lesions reveal about disease and the best use of imaging measures in clinical trials.
The interpretive proton dance—led by the first speaker, Sean Deoni, a Brown University neuroscientist who studies myelination patterns in human development—was definitely an early highlight of the meeting. The idea was to illustrate T1 and T2 relaxation—that is, the time it takes for protons to return to equilibrium (T1) and fall out of sync (T2) after undergoing a magnetic pulse from an MRI machine. The lesson was useful for those like me who are not intimately familiar with the physics behind these two ubiquitous imaging terms. In this subatomic mimicry exercise, we held our hands in the air to imitate the position of protons that have found their way back to magnetic equilibrium (T1) and then bent our torsos 90 degrees and rotated to become right angles that quickly became misaligned as they dephased (T2). T1 and T2 reflect the spin characteristics primarily of protons in water, properties that depend strongly on the molecules associated with the water—such as the fat in myelin—but neither measure is specific for myelin. Deoni outlined his work on imaging normal myelin development in children ages 0–5 years and described a method for approximating the myelin component from these parameters. The need for a myelin-specific imaging approach was discussed several times during the meeting.
Probably the newsiest talk at the meeting detailed the use of high-resolution MRI to track individual lymphocyte migration in the bloodstream. Erik Shapiro, a radiologist at Yale University who gave the talk, stressed that he had never tried the technique in MS, but its single-cell resolution might give a glimpse of the early disease stages, when lymphocytes first breach the blood-brain barrier and infiltrate the brain. A handful of groups during the past decade have used so-called ultrasmall superparamagnetic iron oxide (USPIO) particles to help detect lesions in animal models of MS as well as in humans with the disease; monocytes take up the particles, which show up on MRI scans because they are magnetic. The particle Shapiro’s group is developing, however, gives off a signal that’s powerful enough to allow detection of individual immune cells. Because the particles are so magnetically robust, labeled immune cells could be imaged with high-resolution MRI before abnormalities show up with conventional MS imaging methods or possibly even before symptoms appear, Shapiro later explained to MS Discovery Forum. When he and his colleagues injected labeled cells into a mouse model of stroke, more cells crossed into the brain than in control animals, indicating an immune response. About two-thirds of the dark spots on the animals’ brain scans consisted of individual cells; the other spots included two or three cells. Shapiro said in his talk, however, that scaling up from mice to humans could pose some challenges, such as maintaining single-cell resolution in a larger brain. Furthermore, no such USPIO particles are currently FDA-approved for cell labeling, although one was recently approved for treating anemia. Researchers at the NIH recently reported that this antianemia iron oxide particle can combine with other compounds and be used off-label to track single immune cells in mice (Thu et al., 2012).
One thing that struck me, a relative newcomer to MS research, was how many significant issues remain unresolved in interpreting observable features of the disease. For example, Elizabeth Fisher, a biomedical engineer at the Cleveland Clinic’s Lerner Research Institute in Ohio, described the poorly understood relation between lesions and atrophy in the MS brain. Although both pathological markers are central elements in the disease, researchers have struggled to find a correlation between them. “There seems to be some aspect of neurodegeneration that is not related to the lesion but sort of has a life of its own,” she said—as if there’s a feedback loop in which atrophy begets more atrophy. Fisher’s group showed that white matter lesions account for only about a quarter of subsequent atrophy, although the correlation was tighter between gray matter lesions—that is, those located in the cerebral cortex—and atrophy that developed later. [Gray matter lesions are increasingly considered to play a major role in disease pathology (see “More Than Meets the Eye”).] About 20% of MS brains don’t seem to have macroscopic lesions at all, Fisher noted, yet they undergo severe atrophy.
In response to a question after her talk, she said that previous efforts to predict progression from degree of atrophy weren’t successful, adding that recent imaging advances might make it worth taking another look at that question. “I think one of the reasons we see a big disconnect is that we are not really detecting [all] cortical lesions,” she said.
In his talk, Klaus Schmierer, a neurologist at Barts and the London School of Medicine and Dentistry, pointed out some of the reasons that imaging gray matter lesions has proven so challenging. They tend to be smaller than white matter lesions, plus the lower myelin content of gray matter decreases contrast on an MRI scan; what’s more, gray matter lesions seem to remyelinate more extensively than white matter lesions, so they might become less visible or disappear over time. Fisher stressed in her talk that applying new and increasingly powerful imaging methods, such as 7T MRI, would help paint a more accurate picture of how different pathological features develop over time and how they might connect.
Many of the talks focused on techniques beyond the standard clinical imaging of T1, T2, and gadolinium-enhanced lesions. Matilde Inglese, a neurologist at Mount Sinai School of Medicine in New York City, discussed her own and others’ work on imaging N-acetylaspartate (NAA), an amino acid derivative that is the second-most-abundant metabolite in the brain. NAA dwells exclusively in neurons, so researchers think it somehow marks axonal integrity and viability. MS lesions have a reduced concentration of the molecule relative to normal brain tissue—a decline that has generally been assumed to reflect neuroaxonal loss. But if that were the whole story, Inglese said in her talk, then a drop in NAA should closely correlate with a decrease in brain volume, which isn’t always the case.
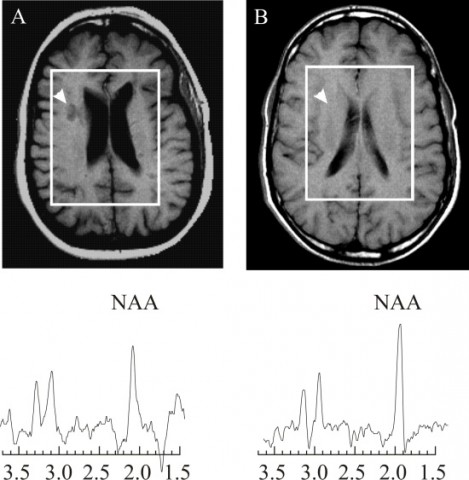
NAA is synthesized in mitochondria, which supply energy to the cell, and Inglese described work from her lab and others suggesting that part of the NAA signal decrease reflects mitochondrial dysfunction and changes in cell metabolism. I had come across mentions of a mitochondrial component of the disease (for example, see “Altered Immunity, Crippled Neurons”), but it’s an area of MS investigation that I didn’t know much about—perhaps because it’s relatively new, appearing on the research radar only in the last decade or so. Demyelinated axons require extra energy to function, studies suggest, and that need reverses after axons remyelinate. Investigators are still unsure about how changes in mitochondria contribute to the disease, but NAA imaging might help shed light on this question.
The second day of the meeting concentrated on translational issues, with speakers and audience members discussing at length the strengths and weaknesses of new and old imaging techniques in diagnosis and prognosis and assessing drug efficacy in clinical trials. Jerry Wolinsky, a neurologist at the University of Texas Health Science Center in Houston, gave the last talk of the meeting, about imaging in clinical trials for neuroprotective drugs. Neuroprotection is one of the field’s next therapeutic frontiers: Unlike agents developed so far, which act primarily on the disease’s immune component, treatments that prevent or reverse neuronal damage might help patients with progressive MS. Reiterating an idea that he and other MS clinical trial experts have kicked around for the past couple of years, Wolinsky said that so-called adaptive, seamless trials that combine Phase II and Phase III testing are the only way to achieve trials that truly measure changes in MS progression (Chataway et al., 2010). Adaptive trials use statistics that allow data analysis at key points during the trial rather than only at the end; researchers can then tweak trial parameters—for example, drop arms where the dosing is off or the treatment is clearly ineffective and rerandomize those patients—while the study is still in progress. He proposed running trials for 5 years or more and using imaging measures of lesions and atrophy early on but ending with measures that demonstrate clinical improvement, such as the Expanded Disability Status Scale. “Finding good neuroprotective drugs is really going to be the issue,” he said. “I think we have to keep our eyes and ears out in other fields.” Some agents—such as the Alzheimer disease medicine memantine—that improve nerve function and are approved for other indications have been tested in MS, although successes have not yet emerged from this approach.
Key open questions
- What techniques can be developed to specifically image myelin in the brain?
- What insights into MS can imaging markers of very early disease reveal?
- What approaches might improve gray matter imaging?
- What is the association between MS lesions and brain atrophy?
- Do changes in N-acetylaspartate concentration reflect disease-related energetic effects?
- What newer imaging techniques should be validated for use as clinical measures?
Image credits
Thumbnail on landing page. Courtesy of Matilde Inglese, Mount Sinai School of Medicine, New York City.
Fig. 1. Courtesy of Matilde Inglese, Mount Sinai School of Medicine, New York City.



Comments
Thanks for covering the meeting. I definitely agree that we need to improve our ability to image certain aspects of MS-related pathology, including cortical lesions and demyelination. But as we're learning more about how the disease changes over time, it's becoming clear that we need to focus on different aspects of the disease at different time points. For example, what's important during recovery from relapse - how a newly developed lesion repairs itself - may be quite different from what's important when relapses are no longer happening, or when someone is being successfully treated with disease-modifying therapies. This has major implications for how we select participants in clinical trials and how we will assess prognosis and response to treatment in the future.