Remyelination: Are Exosomes Containing microRNA the Answer?
Naturally produced exosomes with miRNA led to increased myelination in vitro and in vivo
Today’s treatments for multiple sclerosis aim squarely at the immune system, not the sheath of myelin that insulates and nourishes axons. Eventually, MS destroys that protective layer formed by oligodendrocytes for good. But that could soon change with a new therapeutic strategy to remyelinate axons, currently under investigation by Richard Kraig, M.D., Ph.D., at the University of Chicago, Illinois.
Kraig’s team showed that naturally produced nano-sized vesicles called exosomes packed with microRNA (miRNA) led to increased myelination in healthy rats and remyelination in an in vitro model of demyelination. Kraig presented the work at the 2013 annual meeting of the Society for Neuroscience in San Diego, California. At a press conference there dedicated to MS research, he called the tiny vesicles “Mother Nature’s way of healing the brain.”
“The findings shown today represent real promise for the millions suffering from MS,” said press conference moderator Jeffrey Rothstein, M.D., Ph.D., who studies neurodegenerative disease at Johns Hopkins University in Baltimore, Maryland, and was not involved in the work. Despite many remaining questions, he told MSDF in an email, “miRNA represents a novel approach to [MS] therapeutics that certainly should be considered.”
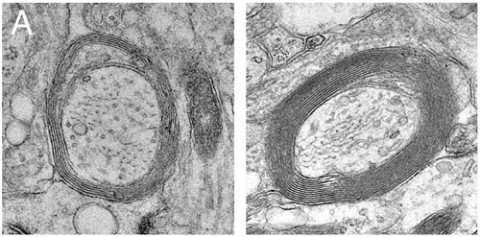
Hippocampal slice cultures
In the new study, published January 15 in the Journal of Neuroimmunology (Pusic et al., 2014), the researchers began by isolating and culturing immune dendritic cells (DCs) from rats’ bone marrow. DCs treated with low levels of the cytokine interferon γ (IFNγ) released exosomes packed with RNA. They then isolated the exosomes from the cultured DC cells and tested them on hippocampal brain slice cultures, an ex vivo system that preserves cellular architecture and interactions.
“When we applied the [IFNγ-stimulated] exosomes to the slice cultures, we saw increased myelin and increased oxidative tolerance to menadione stress,” first author Aya Pusic told MSDF. Pusic is a Ph.D. candidate in Kraig’s lab. Those findings were confirmed by imaging with electron microscopy and immunoblotting for myelin basic protein and reactive oxygen species. Exosomes from unstimulated DCs did not improve myelination, nor did ultraviolet light-exposed exosomes, suggesting that the increase depended on RNA species within the 50-nanometer vesicles.
Would the findings actually translate into increased myelination in animals? Three days after intranasal delivery of stimulated exosomes to healthy aged rats, myelination increased significantly in the cortex compared to unstimulated exosomes. In an in vitro model of demyelination, the authors applied toxic lysolecithin to the slice cultures. Five days later, slices treated with exosomes were significantly remyelinated compared to untreated slices.
Next, the team wanted to identify the molecules inside the exosomes that promoted myelination and bolstered cells against oxidative stress. Using microarrays, they compared the miRNAs found in IFNγ-stimulated and -unstimulated DC exosomes. The stimulated exosomes were enriched in miRNAs known to contribute to oligodendrocyte precursor cell (OPC) differentiation and myelination, most notably miR-219. Several miRNAs involved in anti-inflammatory responses were also found in stimulated exosomes.
Once miRNA reaches its cellular destination, it targets intracellular messenger RNA (mRNA) to regulate protein production. The team next wanted to determine whether the IFNγ-stimulated exosomes would indeed alter expression of genes targeted by the miR-219 contained inside. After applying exosomes to slice cultures, expression of two major miR-219 targets declined: PDGFRα, a mitogen receptor that, when activated, tips cells toward proliferation and away from differentiation; and ELOVL7, a regulator of lipid and oxidative metabolism. This gene regulation, the authors deduced, would encourage OPCs to become myelinating oligodendrocytes.
To see whether the exosomes could drive differentiation of OPCs, the researchers isolated OPCs in a dish and treated them either with stimulated exosomes or with a mimic of miR-219. Compared to controls, both treatments increased differentiation of OPCs into oligodendrocytes, according to cell stage-specific markers.
Would such a boost in differentiation deplete the progenitor population? Dwight Bergles, Ph.D., who studies OPCs at Johns Hopkins University and was not involved in the study, told MSDF that he would not expect OPC depletion. “OPCs will proliferate to maintain homeostasis. When any cells are removed—due to differentiation or death, for example—that creates a void in the population. OPCs try to keep their density constant; if they differentiate, you’ll see proliferation,” Bergles said. Kraig’s findings confirmed that: Staining for NG2, a marker of OPCs, did not differ between slices treated with IFNγ-stimulated or -unstimulated DC exosomes.
Net effect beneficial?
To see what types of cells took up the exosomes, the researchers attached a nanoparticle to the vesicles that allowed them to track them after they were applied to cultured slices. No neurons contained the labeled vesicles; the majority of IFNγ-stimulated exosomes were found in oligodendrocytes, as well as some in microglia and astrocytes. “I wonder what the effects would be in those other cell types,” Bergles said. “MicroRNAs are not unique to oligodendrocytes, and who knows what effects they might have in other glia. One would just hope that the net effect would be beneficial.”
Kraig’s work is part of a push by the U.S. National Institutes of Health (NIH) to understand extracellular RNA (exRNA). Danilo Tagle, Ph.D., associate director for special initiatives at the U.S. National Center for Advancing Translational Sciences (NCATS) in Bethesda, Maryland, heads the cross-divisional team directing 24 research projects, including Kraig’s, in the Extracellular RNA Communication program. “The project is aimed particularly at regulatory RNA, which might have the biggest impact on cells’ biology and in translational science,” Tagle told MSDF. The program, funded by the NIH’s Common Fund, is intended to cut across disciplines to better understand the unappreciated potential of exRNA and rapidly translate that potential to the clinic.
Snippets of messenger ribonucleic acid (mRNA) carry the genetic directive for construction of all our cells’ proteins. Until recently, noncoding RNA—all the other tiny bits with unknown function—were thought of as “junk.” But a new understanding of miRNA has caused scientists to rethink these tiny molecules that can turn expression of other genes on and off.
Outside of cells, enzymes rapidly degrade RNA, so scientists had long assumed that RNA was confined—at least functionally—to the cytoplasm. Now researchers have found that RNA can travel far and wide, affecting genes in other cells. In order to survive the hostile extracellular environment, miRNA travels through the body protected—either bound to lipoproteins or encapsulated within exosomes.
Only recently have researchers been able to study the biological roles of exosomes, even though they were identified decades ago using electron microscopy, Tagle told MSDF: “At that time, they were mistaken for cellular debris.” But now, with the availability of high-throughput sequencing technology, researchers have the ability to peer inside at the contents of the far-ranging signaling packets. “Now we know exosomes carry a rich and diverse cargo,” Tagle said. That cargo includes regulatory miRNAs, messenger RNAs, and proteins.
Exosomes are naturally released by every cell type and are found in bodily fluids including blood, cerebrospinal fluid, and urine. Because the particles are endogenous, they are not detected by the body’s circulating immune system. Each cell type produces a unique signature of exosome contents, and this profile changes under different conditions, such as age or cellular stress. Those properties poise exosomes to treat—and track—conditions ranging from neurodegenerative diseases to cancer and diabetes.
For example, Howard Weiner, an MS researcher at Brigham and Women’s Hospital and Harvard Medical School in Boston, Massachusetts, also received a grant as part of the Extracellular RNA Communication program and has been working to develop biomarkers of MS disease progression based on miRNAs (see related MSDF story).
Whether exosomes containing miR-219 will lead to improvement in animals with experimental autoimmune encephalomyelitis (EAE) or in patients with MS remains to be seen. Myelination results from a series of different events: cell mobilization, differentiation, and wrapping. In both MS and EAE, Bergles pointed out, we do not know exactly where the remyelination failure occurs. Nevertheless, Bergles said, “if you could deliver something to get them over that hump, it could provide a real benefit.”
Tagle and Kraig are optimistic that exosomes could do just that in MS patients, and they hope to see human clinical trials starting 5 years from now—“or sooner,” Tagle said.
Key open questions
The exosomes spurred remyelination in demyelinated slice cultures; what will their effect be on remyelination in animals with EAE and in people with MS?
Would increased remyelination in MS lead to disease modification or improvement in symptoms, or might remyelination have unintended consequences?
What could exosome delivery in animals or humans teach us about the disease course of MS?
Disclosures and sources of funding
The work was supported by funding from the NIH, including the NIH Common Fund, NCATS, the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, the White Foundation, the National Institute of General Medicine, and the National Multiple Sclerosis Society. The authors and commenters had nothing to disclose.


