Innate Differences in Demyelination
In a study that used two colors to distinguish the cell types in a mouse model of multiple sclerosis, invading macrophages appear to be stripping myelin from axons, while resident microglia are virtually shut down
 When a mob converges on a scene of impending destruction, it’s often hard to tell which individuals cause trouble and which ones may be innocent bystanders or even potential heroes. A new study provides the clearest picture yet that look-alike innate immune cells crowding around doomed myelin have distinct roles in disease.
When a mob converges on a scene of impending destruction, it’s often hard to tell which individuals cause trouble and which ones may be innocent bystanders or even potential heroes. A new study provides the clearest picture yet that look-alike innate immune cells crowding around doomed myelin have distinct roles in disease.
In two types of images taken on day one of experimental autoimmune encephalomyelitis (EAE), an MS-like condition in mice, researchers caught invading monocytes from the blood red-handed in a direct attack on myelin. Meanwhile, the resident microglia of the central nervous system (CNS), which had also rushed to the scene, appeared to be nearly shut down. These two distinct groups of cells are both unhelpfully called macrophages and have been virtually indistinguishable in an inflamed CNS until recently.
“It’s a fantastic advance,” said Dwight Bergles, Ph.D., a neuroscientist at Johns Hopkins University, who was not involved in the study. “This study took advantage of genetics to paint the different populations of cells with different colors and unambiguously discriminate the different characteristics. It suggests they have different roles in disease. This is the beginning of a new series of studies.”
The findings were published in a cover article in the July 28 Journal of Experimental Medicine, by a team in the lab of Richard Ransohoff, M.D., a neurologist at the Cleveland Clinic in Ohio (Yamasaki et al., 2014).
“This paper sets a new standard for further studies in the field,” wrote Michael Heneka, M.D., a neurologist at the University of Bonn in Germany, in a commentary that accompanied the paper (Heneka, 2014). “For the first time, MDMs [monocyte-derived macrophages] and MiDMs [microglia-derived macrophages] have been clearly differentiated and the morphological relationship to axoglial structures has been analyzed.”
Twin sons of different mothers
The brain’s innate immune cells were first identified almost 100 years ago, but only in the past several years have researchers confirmed that microglia arise at a different time and place in development than monocytes, the innate immune cells circulating in the blood that invade an inflamed CNS and mingle with the microglia. At the same time, other recent studies have reported, microglia have unexpected properties beyond their macrophage duties of surveillance and clearing debris and are uniquely crucial for normal healthy brains.
In MS, researchers have reasoned, the different origins and normal functions mean the two groups of cells may also have different roles in pathology. “Microglia and monocytes live parallel lives during adulthood, but they can meet in an inflamed brain as macrophages,” said Ransohoff, a senior author of the paper, at a meeting on glia at Cold Spring Harbor Laboratory in New York in July. “They will look alike, but they will not be alike.”
Scientists have developed elaborate methods to try to tell the cells apart in mice, but with unsatisfying results. In the new study, Ransohoff and his colleagues asked whether the pathological roles of the cells could be traced if they were labeled with red and green fluorescent proteins in the same mice.
“We were lucky enough to find a recently developed model that tagged the different myeloid cells with different fluorochrome markers [and] could be used to discriminate between MDMs and MiDMs right at the onset of experimental autoimmune encephalitis,” Ransohoff told MSDF. “If one looks at an active MS lesion, one sees a tremendous infiltration of macrophages, which are clearly involved in the removal of myelin, which is the fundamental problem in MS.”
The start of MS remains a mystery in people, but in mice the first day of disease onset occurs predictably 18 days after the animals are immunized to create EAE. In the spinal cords of mice with green-labeled microglia and red-labeled monocytes, the researchers found equal numbers of cells at EAE onset, although the cell population dynamics differed before and after. The team analyzed other telling features, including the shape and size of cells, as well as the cells’ waving arms, known as processes.
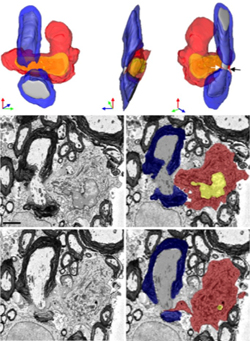
Armed with those structural distinctions, the researchers turned to a serial block-face scanning electron microscopy technique to identify individual cells of each type interacting with a length of myelin-wrapped axon. The technique images the surface of a block of tissue, scrapes off a thin layer, and images the new surface. The images are reconstructed in a three-dimensional model. Electron microscopy provides exquisite details not possible with light microscopy, but there is no good way yet to label cells for serial block-face method.
“To make it simple, monocytes were the bad guys, attacking myelin and removing it from axons, even where the axons appeared perfectly healthy and normal,” Ransohoff told MSDF. “The myelin appeared normal where it was not being pulled off by monocytes. We never found microglia attacking myelin directly.”
News on nodes
In other intriguing findings, the monocytes seemed to attach preferentially at the ends of myelin segments, known as nodes of Ranvier. The nodes frame a stretch of unwrapped axon where sodium channels can conduct impulses.
The complicated and delicate structure of the nodes may be particularly vulnerable to a monocyte attack, Ransohoff speculated: “We are now very intent on figuring out the monocyte signals.”
He wondered whether the monocytes were initiating or responding to a pathological change in the nodes. To help answer the question, the team eliminated most of the myelin-eating monocytes by doubling up on the red protein label. In these tagged mice, the fluorescent markers are knocked into one allele of one key gene used by one cell type and not the other, effectively replacing its functional protein with a glowing color. A double-red knock-in eliminated both copies of the CCR2 protein in monocytes, which is necessary for them to invade the CNS during immune-mediated inflammation.
“It was a beautiful strategy,” said Bergles, who co-chaired the Cold Spring Harbor glia meeting in July. The knock-in approach is challenging, costly, and very clean, he said. It doesn’t work perfectly. The study authors found one monocyte glommed onto a node in the double knock-in mice, compared to five in an equivalent set of tissues from the red-green reporter mice. But despite the scarcity of demyelinating monocytes and the delayed disease onset, the nodes still showed some disrupted pathology in the electron microscope. These images were taken one day before onset, when a sharp weight drop in the mice precedes overt EAE.
Microglial questions
A surprise for Bergle came from the gene expression analysis of microglia on that first day of disease onset, as compared to microglia from a healthy brain. Although microglia had turned on some genes needed to move, sense signals, and migrate, many more genes were dialed down in an overall repression of metabolism and activation.
“We have strong preliminary data that the gene expression decreases we’ve already seen are associated with DNA methylation changes, so they may be quite long lasting,” Ransohoff told MSDF. “We want to follow through to see if mice recover and remyelinate and to see if the microglia recover their ability to contribute to repair.”
Another question that arises about microglial function comes from evidence for an important but undefined role that microglia play in promoting EAE. In one paper from the lab of Marco Prinz, M.D., at the University of Freiburg in Germany (Goldmann et al., 2013), mice missing an inflammatory signaling protein only in microglia were nearly resistant to EAE.
“If you put his work together with ours, it’s a fascinating open question,” Ransohoff told MSDF. “OK, the microglia [in our EAE mice] look very repressed and nonfunctional. But a microglia inflammatory response is necessary for EAE to occur. It suggests that, before onset, microglia are doing something that makes tissue able to support the EAE process. We’re going to go after that.”
In his commentary, Heneka said the findings needed to be verified in people and extended over time points in the entire disease course in mice to track the fate and behavior of the cells in disease. Bergles, whose lab published a dynamic imaging study of oligodendrocyte precursor cells last year (Hughes et al., 2013), is enthusiastic about the potential applications of the two-color methodology to visualize cell behavior in living tissues.
The findings may be more broadly applicable, but the relative roles of monocytes and microglia in inflammatory conditions of the brain and spinal cord may vary with the specific disease, such as stroke, traumatic injury, or Alzheimer’s, Ransohoff said.
“This study makes it almost imperative that similar tools be used to in other neurodegenerative diseases,” Joseph El Khoury, M.D., a neuroimmunologist at Massachusetts General Hospital in Boston, said. “This is just the beginning of dissecting the role of each of these cells in pathology.”
Key open questions
- Do monocytes recognize disrupted nodes or do monocytes create disrupted nodes?
- How does the behavior of monocytes and microglia change over time in EAE and MS and contribute to disease pathology?
- What epigenetic changes influence the behavior of microglia in EAE and MS?
- What are the relative contribution and specific roles of monocytes and macrophages in other neuroinflammatory conditions?
- What technology can be developed to label proteins and cells for unequivocal identification in serial block-face scanning electron microscopy?
Disclosures and sources of funding
Richard Ransohoff, M.D., a neurologist at the Cleveland Clinic in Ohio, is on MSDF’s scientific advisory board. The research was supported by grants from the U.S. National Institutes of Health, the Charles A. Dana Foundation, the National Multiple Sclerosis Society, and the Williams Family Fund for MS Research, as well as a Postdoctoral Fellowship from the National Multiple Sclerosis Society (to N. Ohno). The authors declare no competing financial interests.


