Early B Cells Found in Gut, Schooled by Microbes
Study in mice shows distinctive antibody repertoire in gut-bred B cells
Although T cells have long dominated the immunology of multiple sclerosis (MS), B cells have recently emerged as major players in disease pathology and therapeutic targets. A new study extends the potential influence of the microbiome on immune function with the discovery of a small but distinctive population of B cells in young mice that acquires its antibody diversity in the intestines under the influence of the bacteria there.
The authors found B cells with evidence of freshly made antibodies in the mouse gut, specifically in the connective tissue surrounding the intestinal walls, the mucosal layer known as the lamina propria. The findings were reported online August 21, 2013, in the journal Nature (Wesemann et al., 2013).
Not only did a population of B cells appear to be developing in the gut, but those cells also appear to spin out a different repertoire of antibodies than those in the bone marrow. The developing B cells in the gut surged at about 3 weeks of age, a time when mice are weaned from their mother’s milk and when gut microbes expand. Finally, the paper reports that exposing germ-free mice to microbes boosted the developing B cells throughout the body, including a 20% increase in the gut's unique population.
"This paper is important because it's talking about how you shape your immune repertoire," said Joan Goverman, Ph.D., an immunologist at the University of Washington, Seattle, who was not involved in the study, in an interview with MSDF. "The big step this paper takes is showing B cell maturation from the earliest stage in the small intestine itself."
The paper implicates another arm of the immune system in the trendy field of commensal microbes and their role in health, pointed out Brown University immunologist Mark Schlissel, M.D., Ph.D., in an accompanying commentary in Nature (Schlissel, 2013). "Differentiation of a type of immune cell called Th17 cells requires the presence of certain bacteria in the gut (Ivanov et al., 2009)," he wrote. "Conversely, mice that have no resident microorganisms have perturbed immune responses and a diminished amount of lymphoid tissue, which is an integral part of the immune system. [The new paper] extends the spectrum of these mutualistic interactions."
The authors and other experts contacted for this story said they do not know the functional significance, if any, of the gut B cells in mice, not to mention the implications for health and disease in mice or people.
"Exons that encode B cell antigen receptor variable regions are being assembled in developing B cells in the gut," said senior author Frederick Alt, Ph.D., an immunobiologist and Howard Hughes investigator at Children's Hospital Boston and Harvard Medical School, whose lab worked out key aspects of the diverse antibody generation process, known as V(D)J recombination, several years ago. "They're being molded. The question is, what are they being molded for?”
V(D)J refers to the variable gene segment, the diversity gene segment, and the joining gene segment. The three segments are combined to create a complete V(D)J exon that encodes the variable antigen-binding region of antibodies.
Antibody repertoire
B cells have many roles, but the new study addresses one of their best known features—creating an enormously diverse repertoire of antibodies that recognize infections not yet encountered but that will tolerate the body's own tissues and ignore nonharmful antigens, such as pollen and food.
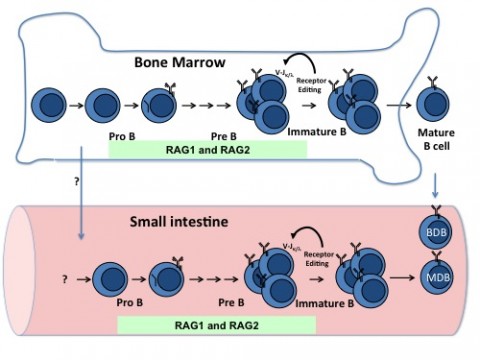
Early in development, millions of new B cells randomly assemble a seemingly endless variation of antibodies by shuffling DNA pieces from three gene segments. The random V(D)J process results in some molecules that react to the body's own antigens. One way to purge self-reactive B cells is called receptor editing, which salvages the B cell by signaling it to continue its V(D)J shuffle until it can produce an acceptable antibody, Alt told MSDF. In mice, as in people, this was thought to happen exclusively in the bone marrow.
First author Duane Wesemann, M.D., now an allergist and clinical immunologist at Brigham and Women's Hospital and Harvard Medical School in Boston, began the study as a postdoctoral fellow with a different mission. He was looking for B cell lymphoma tumor progenitors in the abdominal lymph nodes. A previous study in the Alt lab had shown that B cells undergoing V(D)J recombination—in particular, a redo known as receptor editing—may occur in peripheral tissues, perhaps in the mesenteric lymph nodes, where the tumors were found. He struck out in the lymph nodes. Plenty of mature B cells can be found there, but there was no compelling evidence that they expressed the telltale enzyme that initiates V(D)J recombination, RAG1/RAG2 endonuclease (RAG).
Then Wesemann looked at the problem a different way. He examined other gut tissues for RAG activity at different ages in the mice. Using a mouse model in which the RAG2 protein was fused to a green fluorescence protein, he found a substantial population of RAG-expressing B cells in the small intestinal lamina propria in weaning-aged mice.
In one experiment to further explore this finding, the investigators used ligation-mediated polymerase chain reaction to demonstrate that V(D)J recombination was occurring in the gut. To evaluate the difference in populations of B cells, the researchers used large-scale genomic sequencing to evaluate the heavy- and light-chain immunoglobulin sequences of the antibodies, including the distinctive V(D)J junction points.
In the last key experiment, the team compared B cell development in germ-free mice, which had a reduced microbe load, with germ-free mice that had been colonized by exposure to standard intestinal flora. The addition of microbes increased the amount of developing B cells both in the gut and in the bone marrow. Colonizing germ-free mice, rather than comparing them to mice whose immune systems developed under normal bacteria conditions, reduces confounding by other deficits in the immune system caused by the lack of microbes, Goverman told MSDF, praising the design.
There's a certain scientific symmetry to the discovery of developing B cells in the gut of mice and the possibility that they may be found in people. B cells were originally described in the 1960s using chickens as model organisms. They were named for an appendix-like structure, the bursa, not found in people. In birds, sheep, and rabbits, later research showed, B cells primarily develop much of their antibody repertoire in the gut. As in the other animals, a surge of this diversification happens early in life.
But in people and in mice, the “B” in B cells might as well stand for bone marrow, where most of them are still likely to develop. "The idea our study raises is that the receptor-editing process can occur in the gut, where antigens from the gut microbes are available to interact and have a window of opportunity to shape the receptor-editing process," Wesemann said.
In fact, the role of editing in gut B cells is unknown, Alt said. Editing in bone marrow B cells is considered a process to eliminate autoreactive receptors. "While editing could serve a similar role in gut B cells," he said, "by analogy to what happens in other species, we also speculate that editing in the gut may diversify potential antibody repertoires of B cells that develop there."
"This is an intriguing thing to think about," Goverman said. "It's complete speculation, but could this be a way of explaining how susceptibility to certain autoimmune diseases like MS can be shaped early in life even though the disease is manifested later in life?" She added that it's bound to be even more complicated than it now appears.
Key open questions
- What role, if any, do gut-bred B cells play in immune homeostasis in mice?
- What distinguishes B cells that develop in the gut from those in the bone marrow?
- What is the source of developing B cells in the gut? Do they come from noncommitted B cell progenitors getting there? If so, at which developmental stage?
- Do developing B cells also undergo a primary diversification in gut tissue of people? If so, what is the role of those B cells in health and disease?
- Do developing gut B cells contribute to B cell lymphomagenesis?
Disclosures
This work was supported by the National Institutes of Health, the American Academy of Allergy Asthma and Immunology, CSL Behring, a Career Award for Medical Scientists from the Burroughs Wellcome Fund, and the Howard Hughes Medical Institute.


